Development and Validation of a Stability Indicating RP-HPLC Method for Simultaneous Estimation of Teneligliptin and Metformin
- PMID: 32454773
- PMCID: PMC7227910
- DOI: 10.4274/tjps.galenos.2018.16768
Development and Validation of a Stability Indicating RP-HPLC Method for Simultaneous Estimation of Teneligliptin and Metformin
Abstract
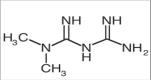
Objectives: The main objective of the present work is to develop a simple, precise, specific and stability method indicating reverse phase high performance liquid chromatography method for simultaneous estimation of teneligliptin and metformin in bulk and tablet dosage form.
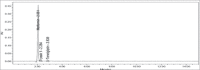
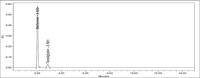
Materials and methods: The analysis was performed with a Kromasil C18 column (250×4.6 mm, 5 μm) at 30°C using buffer: acetonitrile: methanol (65:25:10, v/v/v) as mobile phase. The detection was carried out with a flow rate of 1.0 mL/min at 254 nm.
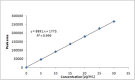
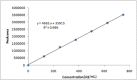
Results: The retention time of teneligliptin and metformin was 2.842 min and 2.017 min, respectively. The linearity range was 5-30 μg/mL for teneligliptin and 125-750 μg/mL for metformin. The forced degradation studies were performed as per the guidelines of the The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use under acidic, alkaline, oxidative, thermal, photostability, and neutral conditions.
Conclusion: This method was successfully validated for all the parameters and could detect the the correct amounts of active drug substance in formulations that are available in the market. This developed method in the present study could be successfully employed for the simultaneous estimation of teneligliptin and metformin in bulk and tablet dosage form.
Keywords: RP-HPLC; Teneligliptin; metformin; stability studies; validation.
©Copyright 2020 Turk J Pharm Sci, Published by Galenos Publishing House.
Conflict of interest statement
Conflicts of interest: No conflict of interest was declared by the authors. The authors alone are responsible for the content and writing of this article.
Figures





References
-
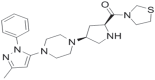
- Yoshida T, Akhashi F, Sakashita H, Kitajima H, Nakamura M, Sonda S, Takeuchi M, Tanaka Y, Ueda N, Sekiguchi S, Ishige T, Shima K, Nabeno M, Abe Y, Anabuki J, Soejima A, Yoshida K, Takashina Y, Ishii S, Kiuchi S, Fukuda S, Tsutsumiuchi R, Kosaka K, Murozono T, Nakamaru Y, Utsumi H, Masutomi N, Kishida H, Miyaguchi I, Hayashi Y. Discovery and preclinical profile of teneligliptin (3-[(2S,4S)-4-[4-(3-methyl-1-phenyl-1H-pyrazol-5-yl)piperazin-1-yl]pyrrolidin-2-ylcarbonyl]thiazolidine): a highly potent, selective, long-lasting and orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Bioorg Med Chem. 2012;20:5705–5719. - PubMed
-
- No authors listed. Indian Pharmacopoeia, Government of India, Ministry of Health & Family Welfare, Volume-2. Ghaziabad; the Indian Pharmacopoeia Commission. 2007:1358–1359.
-
- Merck. The Merck Index (13th ed) White House Station; Merck & Co Inc. 2001;1061.
-
- Sweetman S. ed. Martindale: The Complete Drug Reference Vol. 1. (36th ed) London; Pharmaceutical Press. 2009:453–454.
LinkOut - more resources
Full Text Sources
