Exposure to Air Pollution Exacerbates Inflammation in Rats with Preexisting COPD
- PMID: 32454790
- PMCID: PMC7231193
- DOI: 10.1155/2020/4260204
Exposure to Air Pollution Exacerbates Inflammation in Rats with Preexisting COPD
Abstract
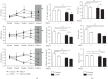
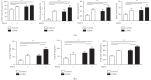
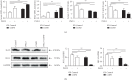
Particulate matter with an aerodynamic diameter equal or less than 2.5 micrometers (PM2.5) is associated with the development of chronic obstructive pulmonary disease (COPD). The mechanisms by which PM2.5 accelerates disease progression in COPD are poorly understood. In this study, we aimed to investigate the effect of PM2.5 on lung injury in rats with hallmark features of COPD. Cardinal features of human COPD were induced in a rat model by repeated cigarette smoke inhalation and bacterial infection for 8 weeks. Then, from week 9 to week 16, some of these rats with COPD were subjected to real-time concentrated atmospheric PM2.5. Lung function, pathology, inflammatory cytokines, oxidative stress, and mucus and collagen production were measured. As expected, the COPD rats had developed emphysema, inflammation, and deterioration in lung function. PM2.5 exposure resulted in greater lung function decline and histopathological changes, as reflected by increased Mucin (MUC) 5ac, MUC5b, Collagen I, Collagen III, and the profibrotic cytokine α-smooth muscle-actin (SMA), transforming growth factor- (TGF-) β1 in lung tissues. PM2.5 also aggravated inflammation, increasing neutrophils and eosinophils in bronchoalveolar lavage fluid (BALF) and cytokines including Interleukin- (IL-) 1β, granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-4. The likely mechanism is through oxidative stress as antioxidants levels were decreased, whereas oxidants were increased, indicating a detrimental shift in the oxidant-antioxidant balance. Altogether, these results suggest that PM2.5 exposure could promote the development of COPD by impairing lung function and exacerbating pulmonary injury, and the potential mechanisms are related to inflammatory response and oxidative stress.
Copyright © 2020 Jing Wang et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



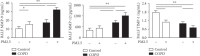
References
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. - PMC - PubMed
-
- Eisner M. D., Anthonisen N., Coultas D., et al. An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2010;182(5):693–718. doi: 10.1164/rccm.200811-1757ST. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

