Adhesion of Hydroxyapatite Nanoparticles to Dental Materials under Oral Conditions
- PMID: 32454927
- PMCID: PMC7222588
- DOI: 10.1155/2020/6065739
Adhesion of Hydroxyapatite Nanoparticles to Dental Materials under Oral Conditions
Abstract
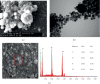
Hydroxyapatite nanoparticles (nano-HAP) are receiving considerable attention for dental applications, and their adhesion to enamel is well established. However, there are no reports concerning the effects of HAP on other dental materials, and most of the studies in this field are based on in vitro designs, neglecting the salivary pellicle-apatite interactions. Thus, this in situ pilot study aims to evaluate the effects of three hydroxyapatite-based solutions and their interactions with different dental material surfaces under oral conditions. Hence, two volunteers carried intraoral splints with mounted samples from enamel and from three dental materials: titanium, ceramics, and polymethyl-methacrylate (PMMA). Three HAP watery solutions (5%) were prepared with different shapes and sizes of nano-HAP (HAP I, HAP II, HAP III). After 3 min of pellicle formation, 10 ml rinse was performed during 30 sec. Rinsing with water served as control. Samples were accessed immediately after rinsing, 30 min and 2 h after rinsing. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used to characterize the particles, and SEM evaluated the pellicle-HAP interactions. SEM and TEM results showed a high variation in the size range of the particles applied. A heterogeneous HAP layer was present after 2 h on enamel, titanium, ceramics, and PMMA surfaces under oral conditions. Bridge-like structures were visible between the nano-HAP and the pellicle formed on enamel, titanium, and PMMA surfaces. In conclusion, nano-HAP can adhere not only to enamel but also to artificial dental surfaces under oral conditions. The experiment showed that the acquired pellicle act as a bridge between the nano-HAP and the materials' surface.
Copyright © 2020 Cíntia Mirela Guimarães Nobre et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures








Similar articles
-
Hydroxyapatite-Based Solution as Adjunct Treatment for Biofilm Management: An In Situ Study.Nanomaterials (Basel). 2021 Sep 21;11(9):2452. doi: 10.3390/nano11092452. Nanomaterials (Basel). 2021. PMID: 34578769 Free PMC article.
-
Oral astringent stimuli alter the enamel pellicle's ultrastructure as revealed by electron microscopy.J Dent. 2017 Aug;63:21-29. doi: 10.1016/j.jdent.2017.05.011. Epub 2017 Jun 12. J Dent. 2017. PMID: 28619693
-
Effect of Tannic Acid on the Protective Properties of the in situ Formed Pellicle.Caries Res. 2017;51(1):34-45. doi: 10.1159/000451036. Epub 2016 Dec 14. Caries Res. 2017. PMID: 27960156
-
The oral cavity--a key system to understand substratum-dependent bioadhesion on solid surfaces in man.Clin Oral Investig. 2009 Jun;13(2):123-39. doi: 10.1007/s00784-008-0243-3. Epub 2009 Jan 10. Clin Oral Investig. 2009. PMID: 19137331 Review.
-
Surface functionalization of hydroxyapatite nanoparticles for biomedical applications.J Mater Chem B. 2024 Jul 17;12(28):6805-6826. doi: 10.1039/d4tb00551a. J Mater Chem B. 2024. PMID: 38919049 Review.
Cited by
-
Modification of in situ Biofilm Formation on Titanium by a Hydroxyapatite Nanoparticle-Based Solution.Front Bioeng Biotechnol. 2020 Dec 4;8:598311. doi: 10.3389/fbioe.2020.598311. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33344433 Free PMC article.
-
Antimicrobial and Antibiofilm Properties of Hydroxyapatite/Nano-Hydroxyapatite in Preventing Dental Caries: A Systematic Review.Eur J Dent. 2025 Jul;19(3):563-579. doi: 10.1055/s-0045-1802568. Epub 2025 May 1. Eur J Dent. 2025. PMID: 40311636 Free PMC article.
-
The Effect of Formulated Dentin Remineralizing Gel Containing Hydroxyapatite, Fluoride, and Bioactive Glass on Dentin Microhardness: An In Vitro Study.Int J Dent. 2024 Aug 10;2024:4788668. doi: 10.1155/2024/4788668. eCollection 2024. Int J Dent. 2024. PMID: 39376678 Free PMC article.
-
Coupled effect of particle size of the source materials and calcination temperature on the direct synthesis of hydroxyapatite.R Soc Open Sci. 2021 Sep 8;8(9):210684. doi: 10.1098/rsos.210684. eCollection 2021 Sep. R Soc Open Sci. 2021. PMID: 34527274 Free PMC article.
-
Influence of ER-CR-YSGG Laser and Photodynamic Therapy on the Dentin Bond Integrity of Nano-Hydroxyapatite Containing Resin Dentin Adhesive: SEM-EDX, Micro-Raman, Micro-Tensile, and FTIR Evaluation.Polymers (Basel). 2021 Jun 8;13(12):1903. doi: 10.3390/polym13121903. Polymers (Basel). 2021. PMID: 34201060 Free PMC article.
References
-
- Shaffiey S. R., Shaffiey S. F. Surface enamel remineralization by biomimetic nano hydroxyapatite crystals and fluoride ions effects. Journal of Ceramic Processing Research. 2016;17(2):109–112.
-
- Fabritius-Vilpoux K., Enax J., Herbig M., Raabe D., Fabritius H. O. Quantitative Affinity Parameters of Synthetic Hydroxyapatite and Enamel Surfaces In Vitro. Bioinspired, Biomimetic and Nanobiomaterials. 2019;8(2):141–153. doi: 10.1680/jbibn.18.00035. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
