Cardiovascular Safety of Clonidine and Dexmedetomidine in Critically Ill Patients after Cardiac Surgery
- PMID: 32455009
- PMCID: PMC7229561
- DOI: 10.1155/2020/4750615
Cardiovascular Safety of Clonidine and Dexmedetomidine in Critically Ill Patients after Cardiac Surgery
Abstract
Purpose: The aim of this retrospective study was to assess the haemodynamic adverse effects of clonidine and dexmedetomidine in critically ill patients after cardiac surgery.
Methods: 2769 patients were screened during the 30-month study period. Heart rate (HR), mean arterial pressure (MAP), and norepinephrine requirements were assessed 3-hourly during the first 12 hours of the continuous drug infusion. Results are given as median (interquartile range) or numbers (percentages).
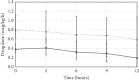
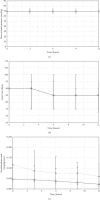
Results: Patients receiving clonidine (n = 193) were younger (66 (57-73) vs 70 (63-77) years, p=0.003) and had a lower SAPS II (35 (27-48) vs 41 (31-54), p=0.008) compared with patients receiving dexmedetomidine (n = 141). At the start of the drug infusion, HR (90 (75-100) vs 90 (80-105) bpm, p=0.028), MAP (70 (65-80) vs 70 (65-75) mmHg, p=0.093), and norepinephrine (0.05 (0.00-0.11) vs 0.12 (0.03-0.19) mcg/kg/min, p < 0.001) were recorded in patients with clonidine and dexmedetomidine. Bradycardia (HR < 60 bpm) developed in 7.8% with clonidine and 5.7% with dexmedetomidine (p=0.51). Between baseline and 12 hours, norepinephrine remained stable in the clonidine group (0.00 (-0.04-0.02) mcg/kg/min) and decreased in the dexmedetomidine group (-0.03 (-0.10-0.02) mcg/kg/min, p=0.007).
Conclusions: Dexmedetomidine and the low-cost drug clonidine can both be used safely in selected patients after cardiac surgery.
Copyright © 2020 Angelina Grest et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Figures

Similar articles
-
Evaluating the Transition From Dexmedetomidine to Clonidine for the Prevention of Withdrawal in Critically Ill Pediatric Patients.J Pediatr Pharmacol Ther. 2020;25(2):104-110. doi: 10.5863/1551-6776-25.2.104. J Pediatr Pharmacol Ther. 2020. PMID: 32071584 Free PMC article.
-
Safety and efficacy of prolonged dexmedetomidine use in critically ill children with heart disease*.Pediatr Crit Care Med. 2012 Nov;13(6):660-6. doi: 10.1097/PCC.0b013e318253c7f1. Pediatr Crit Care Med. 2012. PMID: 22791093
-
Identification of a Conversion Factor for Dexmedetomidine to Clonidine Transitions.J Pediatr Pharmacol Ther. 2024 Aug;29(4):375-378. doi: 10.5863/1551-6776-29.4.375. Epub 2024 Aug 13. J Pediatr Pharmacol Ther. 2024. PMID: 39144393 Free PMC article.
-
[A study of using dexmedetomidine in ventilator bundle treatment in an ICU].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2015 Oct;27(10):836-40. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2015. PMID: 27132448 Clinical Trial. Chinese.
-
Clonidine as a strategy for discontinuing dexmedetomidine sedation in critically ill patients: A narrative review.Am J Health Syst Pharm. 2020 Mar 24;77(7):515-522. doi: 10.1093/ajhp/zxaa013. Am J Health Syst Pharm. 2020. PMID: 32086509 Review.
Cited by
-
Sedation, sleep-promotion, and non-verbal and verbal communication techniques in critically ill intubated or tracheostomized patients: results of a survey.BMC Anesthesiol. 2022 Dec 12;22(1):384. doi: 10.1186/s12871-022-01887-z. BMC Anesthesiol. 2022. PMID: 36503427 Free PMC article.
-
Alpha-2-adrenergic receptor agonists for the prevention of delirium and cognitive decline after open heart surgery (ALPHA2PREVENT): protocol for a multicentre randomised controlled trial.BMJ Open. 2022 Jun 20;12(6):e057460. doi: 10.1136/bmjopen-2021-057460. BMJ Open. 2022. PMID: 35725264 Free PMC article.
References
LinkOut - more resources
Full Text Sources

