Cardiovascular Safety of Clonidine and Dexmedetomidine in Critically Ill Patients after Cardiac Surgery
- PMID: 32455009
- PMCID: PMC7229561
- DOI: 10.1155/2020/4750615
Cardiovascular Safety of Clonidine and Dexmedetomidine in Critically Ill Patients after Cardiac Surgery
Abstract
Purpose: The aim of this retrospective study was to assess the haemodynamic adverse effects of clonidine and dexmedetomidine in critically ill patients after cardiac surgery.
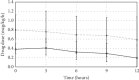
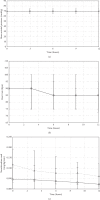
Methods: 2769 patients were screened during the 30-month study period. Heart rate (HR), mean arterial pressure (MAP), and norepinephrine requirements were assessed 3-hourly during the first 12 hours of the continuous drug infusion. Results are given as median (interquartile range) or numbers (percentages).
Results: Patients receiving clonidine (n = 193) were younger (66 (57-73) vs 70 (63-77) years, p=0.003) and had a lower SAPS II (35 (27-48) vs 41 (31-54), p=0.008) compared with patients receiving dexmedetomidine (n = 141). At the start of the drug infusion, HR (90 (75-100) vs 90 (80-105) bpm, p=0.028), MAP (70 (65-80) vs 70 (65-75) mmHg, p=0.093), and norepinephrine (0.05 (0.00-0.11) vs 0.12 (0.03-0.19) mcg/kg/min, p < 0.001) were recorded in patients with clonidine and dexmedetomidine. Bradycardia (HR < 60 bpm) developed in 7.8% with clonidine and 5.7% with dexmedetomidine (p=0.51). Between baseline and 12 hours, norepinephrine remained stable in the clonidine group (0.00 (-0.04-0.02) mcg/kg/min) and decreased in the dexmedetomidine group (-0.03 (-0.10-0.02) mcg/kg/min, p=0.007).
Conclusions: Dexmedetomidine and the low-cost drug clonidine can both be used safely in selected patients after cardiac surgery.
Copyright © 2020 Angelina Grest et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Figures

References
LinkOut - more resources
Full Text Sources

