Contribution of Organofluorine Compounds to Pharmaceuticals
- PMID: 32455181
- PMCID: PMC7240833
- DOI: 10.1021/acsomega.0c00830
Contribution of Organofluorine Compounds to Pharmaceuticals
Abstract
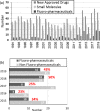
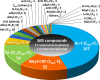
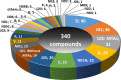
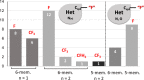
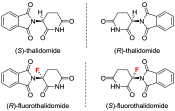
Inspired by the success of fluorinated corticosteroids in the 1950s and fluoroquinolones in the 1980s, fluorine-containing pharmaceuticals, which are also known as fluoro-pharmaceuticals, have been attracting attention for more than half of a century. Presently, about 20% of the commercial pharmaceuticals are fluoro-pharmaceuticals. In this mini-review, we analyze the prevalence of fluoro-pharmaceuticals in the market and categorize them into several groups based on the chemotype of the fluoro-functional groups, their therapeutic purpose, and the presence of heterocycles and/or chirality to highlight the structural motifs, patterns, and promising trends in fluorine-based drug design. Our database contains 340 fluoro-pharmaceuticals, from the first fluoro-pharmaceutical, Florinef, to the latest fluoro-pharmaceuticals registered in 2019 and drugs that have been withdrawn. The names and chemical structures of all the 340 fluorinated drugs discussed are provided in the Supporting Information.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Small Molecule Medicinal Chemistry: Strategies and Technologies; Czechtizky W., Hamley P., Eds.; John Wiley & Sons, Inc., 2015.
- Das P.; Delost M. D.; Qureshi M. H.; Smith D. Y.; Njardarson J. T. A Survey of the Structures of US FDA Approved Combination Drugs. J. Med. Chem. 2019, 62, 4265–4311. 10.1021/acs.jmedchem.8b01610. - DOI - PubMed
-
- Introduction to Biological and Small Molecule Drug Research and Development: Theory and Case Studies; Ganellin R., Roberts S., Jefferis R., Eds.; Elsevier Ltd., 2013.
- Nelson A. L.; Dhimolea E.; Reichert J. M. Development trends for human monoclonal antibody therapeutics. Nat. Rev. Drug Discovery 2010, 9, 767–774. 10.1038/nrd3229. - DOI - PubMed
-
- Isanbor C.; O’Hagan D. Fluorine in medicinal chemistry: A review of anti-cancer agents. J. Fluorine Chem. 2006, 127, 303–319. 10.1016/j.jfluchem.2006.01.011. - DOI
- Purser S.; Moore P. R.; Swallow S.; Gouverneur V. Fluorine in Medicinal Chemistry. Chem. Soc. Rev. 2008, 37, 320–330. 10.1039/B610213C. - DOI - PubMed
- Wang J.; Sánchez-Roselló M.; Aceña J. L.; Del Pozo C.; Sorochinsky A. E.; Fustero S.; Soloshonok V. A.; Liu H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. 10.1021/cr4002879. - DOI - PubMed
- Ilardi E. A.; Vitaku E.; Njardarson J. T. Data-Mining for Sulfur and Fluorine: An Evaluation of Pharmaceuticals To Reveal Opportunities for Drug Design and Discovery. J. Med. Chem. 2014, 57, 2832–2842. 10.1021/jm401375q. - DOI - PubMed
- Gillis E. P.; Eastman K. J.; Hill M. D.; Donnelly D. J.; Meanwell N. A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. 10.1021/acs.jmedchem.5b00258. - DOI - PubMed
- Swallow S. Fluorine in Medicinal Chemistry. Progress in Medicinal Chemistry. Prog. Med. Chem. 2015, 54, 65–133. 10.1016/bs.pmch.2014.11.001. - DOI - PubMed
-
-
Since the approved drugs are different, depending on the country, such as the US, Japan, and Europe, the numbers of them are different depending on the sources. In the present manuscript, we mainly collected the data from the KEGG (Kyoto Encyclopedia Genes and Genomes) DRUG Database at
- We categorized a “small molecule” by molecular weights around <1500 g/mol (not strictly).
-
-
- Jarvis L. M.The drugs of 2018. C&EN 2019; Vol. 97 ( (3), ), January 21.
- U.S. Food and Drug Administration Home Page, Novel Drug Approvals for 2018. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and... (accessed Feb 13, 2020).
- Mei H.; Han J.; Fustero S.; Medio-Simon M.; Sedgwick D. M.; Santi C.; Ruzziconi R.; Soloshonok V. A. Fluorine-Containing Drugs Approved by the FDA in 2018. Chem. - Eur. J. 2019, 25, 11797–11819. 10.1002/chem.201901840. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

