Development of a Method for Screening and Genotyping of HCV 1a, 1b, 2, 3, 4, and 6 Genotypes
- PMID: 32455199
- PMCID: PMC7240817
- DOI: 10.1021/acsomega.0c00386
Development of a Method for Screening and Genotyping of HCV 1a, 1b, 2, 3, 4, and 6 Genotypes
Abstract
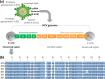
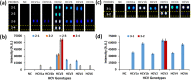
The World Health Organization and the World Health Assembly recommended eradicating hepatitis as a public threat by 2030. The accurate genotyping of hepatitis C virus (HCV) is crucial to achieving this goal because it is vital for the selection of anti-HCV therapy required for complete cure of HCV infection. We report the development of a method for accurate genotyping of HCV 1a, 1b, 2, 3, 4, and 6 genotypes. The merits of the developed method for HCV genotyping include (i) requirement of a single polymerase chain reaction (PCR) primer set, (ii) room-temperature detection in 30 min after the PCR, (iii) no need of highly trained professionals, (iv) highly accurate HCV genotyping results afforded by highly specific DNA-DNA hybridization, and (v) probe sequences that can be used on other platforms.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Assessment of a Novel Automatic Real-Time PCR Assay on the Cobas 4800 Analyzer as a Screening Platform for Hepatitis C Virus Genotyping in Clinical Practice: Comparison with Massive Sequencing.J Clin Microbiol. 2017 Feb;55(2):504-509. doi: 10.1128/JCM.01960-16. Epub 2016 Dec 7. J Clin Microbiol. 2017. PMID: 27927921 Free PMC article.
-
Hepatitis C virus genotypes in French haemophiliacs: kinetics and reappraisal of mixed infections.J Med Virol. 1997 Jan;51(1):36-41. doi: 10.1002/(sici)1096-9071(199701)51:1<36::aid-jmv6>3.0.co;2-t. J Med Virol. 1997. PMID: 8986947
-
[Development and application of a new hepatitis C virus genotyping method with polymerase chain reaction-reverse blot dot technique].Zhonghua Liu Xing Bing Xue Za Zhi. 2005 Jun;26(6):440-3. Zhonghua Liu Xing Bing Xue Za Zhi. 2005. PMID: 16185461 Chinese.
-
Performance of 6 HCV genotyping 9G test for HCV genotyping in clinical samples.Virol J. 2018 Jul 11;15(1):107. doi: 10.1186/s12985-018-1017-4. Virol J. 2018. PMID: 29996859 Free PMC article.
-
[Genotyping and the quantification of hepatitis C virus by the melting curves in light cycler].Rinsho Byori. 2004 Feb;52(2):167-71. Rinsho Byori. 2004. PMID: 15027322 Review. Japanese.
Cited by
-
Distinct Characteristic Binding Modes of Benzofuran Core Inhibitors to Diverse Genotypes of Hepatitis C Virus NS5B Polymerase: A Molecular Simulation Study.Int J Mol Sci. 2024 Jul 23;25(15):8028. doi: 10.3390/ijms25158028. Int J Mol Sci. 2024. PMID: 39125602 Free PMC article.
-
Hepatitis C Diagnosis: Simplified Solutions, Predictive Barriers, and Future Promises.Diagnostics (Basel). 2021 Jul 13;11(7):1253. doi: 10.3390/diagnostics11071253. Diagnostics (Basel). 2021. PMID: 34359335 Free PMC article. Review.
-
Virtual screening, optimization design, and synthesis analysis of novel benzofuran derivatives as pan-genotypic HCV NS5B polymerase inhibitors using molecular modeling.BMC Chem. 2025 Jul 4;19(1):199. doi: 10.1186/s13065-025-01575-2. BMC Chem. 2025. PMID: 40615885 Free PMC article.
-
Towards a Rapid-Turnaround Low-Depth Unbiased Metagenomics Sequencing Workflow on the Illumina Platforms.Bioengineering (Basel). 2023 Apr 25;10(5):520. doi: 10.3390/bioengineering10050520. Bioengineering (Basel). 2023. PMID: 37237590 Free PMC article.
References
-
- Smith D. B.; Bukh J.; Kuiken C.; Muerhoff A. S.; Rice C. M.; Stapleton J. T.; Simmonds P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: Updated criteria and genotype assignment web resource. Hepatology 2014, 59, 318–327. 10.1002/hep.26744. - DOI - PMC - PubMed
- Messina J. P.; Humphreys I.; Flaxman A.; Brown A.; Cooke G. S.; Pybus O. G.; Barnes E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 2015, 61, 77–87. 10.1002/hep.27259. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

