Development of a Method for Screening and Genotyping of HCV 1a, 1b, 2, 3, 4, and 6 Genotypes
- PMID: 32455199
- PMCID: PMC7240817
- DOI: 10.1021/acsomega.0c00386
Development of a Method for Screening and Genotyping of HCV 1a, 1b, 2, 3, 4, and 6 Genotypes
Abstract
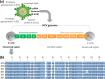
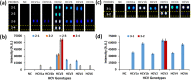
The World Health Organization and the World Health Assembly recommended eradicating hepatitis as a public threat by 2030. The accurate genotyping of hepatitis C virus (HCV) is crucial to achieving this goal because it is vital for the selection of anti-HCV therapy required for complete cure of HCV infection. We report the development of a method for accurate genotyping of HCV 1a, 1b, 2, 3, 4, and 6 genotypes. The merits of the developed method for HCV genotyping include (i) requirement of a single polymerase chain reaction (PCR) primer set, (ii) room-temperature detection in 30 min after the PCR, (iii) no need of highly trained professionals, (iv) highly accurate HCV genotyping results afforded by highly specific DNA-DNA hybridization, and (v) probe sequences that can be used on other platforms.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Smith D. B.; Bukh J.; Kuiken C.; Muerhoff A. S.; Rice C. M.; Stapleton J. T.; Simmonds P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: Updated criteria and genotype assignment web resource. Hepatology 2014, 59, 318–327. 10.1002/hep.26744. - DOI - PMC - PubMed
- Messina J. P.; Humphreys I.; Flaxman A.; Brown A.; Cooke G. S.; Pybus O. G.; Barnes E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 2015, 61, 77–87. 10.1002/hep.27259. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

