Hydroxyethyl Chitosan-Reinforced Polyvinyl Alcohol/Biphasic Calcium Phosphate Hydrogels for Bone Regeneration
- PMID: 32455215
- PMCID: PMC7241017
- DOI: 10.1021/acsomega.0c00727
Hydroxyethyl Chitosan-Reinforced Polyvinyl Alcohol/Biphasic Calcium Phosphate Hydrogels for Bone Regeneration
Abstract
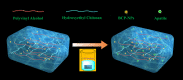
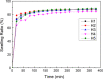
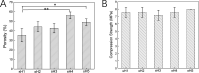
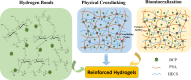
Fabrication of reinforced scaffolds for bone regeneration remains a significant challenge. The weak mechanical properties of the chitosan (CS)-based composite scaffold hindered its further application in clinic. Here, to obtain hydroxyethyl CS (HECS), some hydrogen bonds of CS were replaced by hydroxyethyl groups. Then, HECS-reinforced polyvinyl alcohol (PVA)/biphasic calcium phosphate (BCP) nanoparticle hydrogel was fabricated via cycled freeze-thawing followed by an in vitro biomineralization treatment using a cell culture medium. The synthesized hydrogel had an interconnected porous structure with a uniform pore distribution. Compared to the CS/PVA/BCP hydrogel, the HECS/PVA/BCP hydrogels showed a thicker pore wall and had a compressive strength of up to 5-7 MPa. The biomineralized hydrogel possessed a better compressive strength and cytocompatibility compared to the untreated hydrogel, confirmed by CCK-8 analysis and fluorescence images. The modification of CS with hydroxyethyl groups and in vitro biomineralization were sufficient to improve the mechanical properties of the scaffold, and the HECS-reinforced PVA/BCP hydrogel was promising for bone tissue engineering applications.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







Similar articles
-
Carbon nanotube reinforced polyvinyl alcohol/biphasic calcium phosphate scaffold for bone tissue engineering.RSC Adv. 2019 Nov 28;9(67):38998-39010. doi: 10.1039/c9ra08569f. eCollection 2019 Nov 27. RSC Adv. 2019. PMID: 35540653 Free PMC article.
-
Physicochemical characterization and biocompatibility in vitro of biphasic calcium phosphate/polyvinyl alcohol scaffolds prepared by freeze-drying method for bone tissue engineering applications.Colloids Surf B Biointerfaces. 2012 Dec 1;100:169-76. doi: 10.1016/j.colsurfb.2012.04.046. Epub 2012 May 31. Colloids Surf B Biointerfaces. 2012. PMID: 22766294
-
A Porous Hydrogel with High Mechanical Strength and Biocompatibility for Bone Tissue Engineering.J Funct Biomater. 2022 Sep 3;13(3):140. doi: 10.3390/jfb13030140. J Funct Biomater. 2022. PMID: 36135575 Free PMC article.
-
Development of chitosan/gelatin hydrogels incorporation of biphasic calcium phosphate nanoparticles for bone tissue engineering.J Biomater Sci Polym Ed. 2019 Dec;30(17):1636-1657. doi: 10.1080/09205063.2019.1654210. Epub 2019 Sep 11. J Biomater Sci Polym Ed. 2019. PMID: 31393229
-
Polyvinyl Alcohol-Chitosan Scaffold for Tissue Engineering and Regenerative Medicine Application: A Review.Mar Drugs. 2023 May 17;21(5):304. doi: 10.3390/md21050304. Mar Drugs. 2023. PMID: 37233498 Free PMC article. Review.
Cited by
-
Construction methods and biomedical applications of PVA-based hydrogels.Front Chem. 2024 Feb 15;12:1376799. doi: 10.3389/fchem.2024.1376799. eCollection 2024. Front Chem. 2024. PMID: 38435666 Free PMC article. Review.
-
Advances in Growth Factor Delivery for Bone Tissue Engineering.Int J Mol Sci. 2021 Jan 18;22(2):903. doi: 10.3390/ijms22020903. Int J Mol Sci. 2021. PMID: 33477502 Free PMC article. Review.
-
Fabrication of 3-Dimensional-Printed Bilayered Scaffold Carboxymethyl Chitosan/Oxidized Xanthan Gum, Biphasic Calcium Phosphate for Osteochondral Regeneration.Biomater Res. 2025 Apr 9;29:0186. doi: 10.34133/bmr.0186. eCollection 2025. Biomater Res. 2025. PMID: 40207259 Free PMC article.
-
Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments.Molecules. 2021 May 18;26(10):3007. doi: 10.3390/molecules26103007. Molecules. 2021. PMID: 34070157 Free PMC article. Review.
-
Fabrication of ACP-CCS-PVA composite membrane for a potential application in guided bone regeneration.RSC Adv. 2023 Sep 1;13(37):25930-25938. doi: 10.1039/d3ra04498j. eCollection 2023 Aug 29. RSC Adv. 2023. PMID: 37664206 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

