(Ag)Pd-Fe3O4 Nanocomposites as Novel Catalysts for Methane Partial Oxidation at Low Temperature
- PMID: 32455643
- PMCID: PMC7279560
- DOI: 10.3390/nano10050988
(Ag)Pd-Fe3O4 Nanocomposites as Novel Catalysts for Methane Partial Oxidation at Low Temperature
Abstract
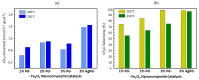
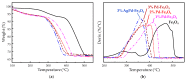
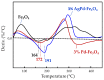
Nanostructured composite materials based on noble mono-(Pd) or bi-metallic (Ag/Pd) particles supported on mixed iron oxides (II/III) with bulk magnetite structure (Fe3O4) have been developed in order to assess their potential for heterogeneous catalysis applications in methane partial oxidation. Advancing the direct transformation of methane into value-added chemicals is consensually accepted as the key to ensuring sustainable development in the forthcoming future. On the one hand, nanosized Fe3O4 particles with spherical morphology were synthesized by an aqueous-based reflux method employing different Fe (II)/Fe (III) molar ratios (2 or 4) and reflux temperatures (80, 95 or 110 °C). The solids obtained from a Fe (II)/Fe (III) nominal molar ratio of 4 showed higher specific surface areas which were also found to increase on lowering the reflux temperature. The starting 80 m2 g-1 was enhanced up to 140 m2 g-1 for the resulting optimized Fe3O4-based solid consisting of nanoparticles with a 15 nm average diameter. On the other hand, Pd or Pd-Ag were incorporated post-synthesis, by impregnation on the highest surface Fe3O4 nanostructured substrate, using 1-3 wt.% metal load range and maintaining a constant Pd:Ag ratio of 8:2 in the bimetallic sample. The prepared nanocomposite materials were investigated by different physicochemical techniques, such as X-ray diffraction, thermogravimetry (TG) in air or H2, as well as several compositions and structural aspects using field emission scanning and scanning transmission electron microscopy techniques coupled to energy-dispersive X-ray spectroscopy (EDS). Finally, the catalytic results from a preliminary reactivity study confirmed the potential of magnetite-supported (Ag)Pd catalysts for CH4 partial oxidation into formaldehyde, with low reaction rates, methane conversion starting at 200 °C, far below temperatures reported in the literature up to now; and very high selectivity to formaldehyde, above 95%, for Fe3O4 samples with 3 wt.% metal, either Pd or Pd-Ag.
Keywords: Ag; EDS; Fe3O4; Pd; Raman; TG in air; TG in hydrogen; XRD; electron microscopy; formaldehyde; heterogeneous catalysis; low-temperature activity; magnetite iron oxide; methane; nanocomposite; oxidation catalysis; palladium; silver.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






References
-
- Hargreaves J.S.J., Hutchings G.J., Joyner R.W. Control of product selectivity in the partial oxidation of methane. Nature. 1990;348:428–429. doi: 10.1038/348428a0. - DOI
-
- Zhao G., Drewery M., Mackie J., Oliver T., Kennedy E.M., Stockenhuber M. The Catalyzed Conversion of Methane to Value-Added Products. Energy Technol. 2019:1900665. doi: 10.1002/ente.201900665. - DOI
-
- Fait M.J.G., Ricci A., Holena M., Rabeah J., Pohl M.M., Linke D., Kondratenko E.V. Understanding trends in methane oxidation to formaldehyde: Statistical analysis of literature data and based hereon experiments. Catal. Sci. Technol. 2019;9:5111–5121. doi: 10.1039/C9CY01055F. - DOI
-
- Smith M.R., Ozkan U.S. The Partial Oxidation of Methane to Formaldehyde: Role of Different Crystal Planes of MoO3. J. Catal. 1993;141:124–139. doi: 10.1006/jcat.1993.1124. - DOI
-
- Amiridis M.D., Rekoske J.E., Dumesic J.A., Rudd D.F., Spencer N.D., Pereira C.J. Simulation of methane partial oxidation over silica-supported MoO3 and V2O5. Aiche J. 1991;37:87–97. doi: 10.1002/aic.690370108. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

