Characteristics of Serotype 3 Invasive Pneumococcal Disease before and after Universal Childhood Immunization with PCV13 in Massachusetts
- PMID: 32455770
- PMCID: PMC7281000
- DOI: 10.3390/pathogens9050396
Characteristics of Serotype 3 Invasive Pneumococcal Disease before and after Universal Childhood Immunization with PCV13 in Massachusetts
Abstract
Background: Although a substantial decline in vaccine-serotype invasive pneumococcal disease (IPD) incidence was observed following the introduction of pneumococcal conjugate vaccines (PCV), the estimated range of thirteen-valent conjugate vaccine (PCV13) effectiveness for serotype 3 disease is wide and includes zero. We assessed the impact of PCV13 on serotype 3 IPD incidence and disease characteristics in Massachusetts' children.
Methods: Serotype 3 IPD cases in children <18 years old were identified via enhanced passive surveillance system in Massachusetts. We compared incidence rates and characteristics of IPD cases before and after PCV13.
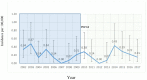
Results: A total of 47 serotype 3 IPD cases were identified from 2002 to 2017; incidence of serotype 3 IPD in the years following PCV13 was 0.19 per 100,000 children compared to 0.21 before PCV 13, incidence rate ratio (IRR) = 0.86 (95% CI 0.47-1.57). The majority (78%) of post-PCV13 serotype 3 IPD cases occurred among fully vaccinated children. Age distribution, clinical syndrome and presence of comorbidities among serotype 3 IPD cases were similar before and after PCV13 introduction. There was no association between the date of the last PCV13 dose and time to IPD to suggest waning of immunity.
Conclusions: seven years following PCV 13 we found no significant changes in serotype 3 IPD incidence or disease characteristics in children in Massachusetts.
Keywords: PCV13; Streptococcus pneumoniae serotype 3; invasive pneumococcal disease.
Conflict of interest statement
Dr. Pelton received research grants to Boston Medical Center for investigator initiated project from Pfizer, Inc and Merck Vaccines. He also received honorarium and consulting fees from Pfizer for participation in advisory boards and consultation and from Merck Vaccines for participation in advisory boards. Kimberly Shea has received investigator-initiated research grants and is a former academic consultant for Pfizer, Inc; and is currently employed by Pfizer, Inc. Dr. Yildirim has received academic investigator-initiated research grants from Merck Vaccines. Dr. Lapidot received an investigator initiated grant to Boston Medical Center from Pfizer, Inc.
Figures
References
-
- Assessment R. Weekly Epidemiological Record Relevé Épidémiologique Hebdomadaire Sommaire. [(accessed on 20 May 2020)];2019 Volume 8:85–104. Available online: https://apps.who.int/iris/bitstream/handle/10665/310968/WER9408.pdf?ua=1.
-
- CDC Pneumococcal Disease Surveillance and Reporting. [(accessed on 10 September 2019)]; Available online: https://www.cdc.gov/pneumococcal/surveillance.html.
-
- Yildirim I., Little B.A., Finkelstein J., Lee G., Hanage W.P., Shea K., Pelton S.I. Surveillance of pneumococcal colonization and invasive pneumococcal disease reveals shift in prevalent carriage serotypes in Massachusetts’ children to relatively low invasiveness. Vaccine. 2017;35:4002–4009. doi: 10.1016/j.vaccine.2017.05.077. - DOI - PubMed
-
- Yildirim I., Hanage W.P., Lipsitch M., Shea K.M., Stevenson A., Finkelstein J., Huang S.S., Lee G.M., Kleinman K., Pelton S.I. Serotype specific invasive capacity and persistent reduction in invasive pneumococcal disease. Vaccine. 2010;29:283–288. doi: 10.1016/j.vaccine.2010.10.032. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources