ASC-Exosomes Ameliorate the Disease Progression in SOD1(G93A) Murine Model Underlining Their Potential Therapeutic Use in Human ALS
- PMID: 32455791
- PMCID: PMC7279464
- DOI: 10.3390/ijms21103651
ASC-Exosomes Ameliorate the Disease Progression in SOD1(G93A) Murine Model Underlining Their Potential Therapeutic Use in Human ALS
Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by progressive degeneration of motoneurons. To date, there is no effective treatment available. Exosomes are extracellular vesicles that play important roles in intercellular communication, recapitulating the effect of origin cells. In this study, we tested the potential neuroprotective effect of exosomes isolated from adipose-derived stem cells (ASC-exosomes) on the in vivo model most widely used to study ALS, the human SOD1 gene with a G93A mutation (SOD1(G93A)) mouse. Moreover, we compared the effect of two different routes of exosomes administration, intravenous and intranasal. The effect of exosomes administration on disease progression was monitored by motor tests and analysis of lumbar motoneurons and glial cells, neuromuscular junction, and muscle. Our results demonstrated that repeated administration of ASC-exosomes improved the motor performance; protected lumbar motoneurons, the neuromuscular junction, and muscle; and decreased the glial cells activation in treated SOD1(G93A) mice. Moreover, exosomes have the ability to home to lesioned ALS regions of the animal brain. These data contribute by providing additional knowledge for the promising use of ASC-exosomes as a therapy in human ALS.
Keywords: MRI; amyotrophic lateral sclerosis; extracellular vesicles; homing; motoneurons; neuromuscular junction; stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
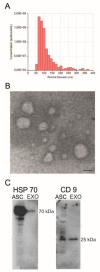





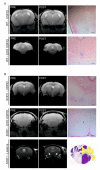
References
-
- Vinsant S., Mansfield C., Jimenez-Moreno R., Moore V.D.G., Yoshikawa M., Hampton T.G., Prevette D., Caress J., Oppenheim R.W., E Milligan C. Characterization of early pathogenesis in the SOD1G93A mouse model of ALS: Part I, background and methods. Brain Behav. 2013;3:335–350. doi: 10.1002/brb3.143. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

