Clinical Implications of DNA Repair Defects in High-Grade Serous Ovarian Carcinomas
- PMID: 32455819
- PMCID: PMC7281678
- DOI: 10.3390/cancers12051315
Clinical Implications of DNA Repair Defects in High-Grade Serous Ovarian Carcinomas
Abstract
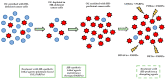
Despite significant improvements in surgical and medical management, high grade serous ovarian cancer (HGSOC) still represents the deadliest gynecologic malignancy and the fifth most frequent cause of cancer-related mortality in women in the USA. Since DNA repair alterations are regarded as the "the Achille's heel" of HGSOC, both DNA homologous recombination and DNA mismatch repair deficiencies have been explored and targeted in epithelial ovarian cancers in the latest years. In this review, we aim at focusing on the therapeutic issues deriving from a faulty DNA repair machinery in epithelial ovarian cancers, starting from existing and well-established treatments and investigating new therapeutic approaches which could possibly improve ovarian cancer patients' survival outcomes in the near future. In particular, we concentrate on the role of both Poly (ADP-ribose) Polymerase (PARP) inhibitors (PARPis) and immune checkpoint inhibitors in HGSOC, highlighting their activity in relation to BRCA1/2 mutational status and homologous recombination deficiency (HRD). We investigate the biological rationale supporting their use in the clinical setting, pointing at tracking their route from the laboratory bench to the patient's bedside. Finally, we deal with the onset of mechanisms of primary and acquired resistance to PARPis, reporting the pioneering strategies aimed at converting homologous-recombination (HR) proficient tumors into homologous recombination (HR)-deficient HGSOC.
Keywords: BRCA reversion mutations; DNA homologous recombination; DNA mismatch repair; DNA repair deficiency; PARP inhibitors; epithelial ovarian cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Schwarz R.F., Ng C.K., Cooke S.L., Newman S., Temple J., Piskorz A.M., Gale D., Sayal K., Murtaza M., Baldwin P.J., et al. Spatial and temporal heterogeneity in high-grade serous ovarian cancer: A phylogenetic analysis. PLoS Med. 2015;12:e1001789. doi: 10.1371/journal.pmed.1001789. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

