Trioxolone Methyl, a Novel Cyano Enone-Bearing 18βH-Glycyrrhetinic Acid Derivative, Ameliorates Dextran Sulphate Sodium-Induced Colitis in Mice
- PMID: 32455822
- PMCID: PMC7287650
- DOI: 10.3390/molecules25102406
Trioxolone Methyl, a Novel Cyano Enone-Bearing 18βH-Glycyrrhetinic Acid Derivative, Ameliorates Dextran Sulphate Sodium-Induced Colitis in Mice
Abstract
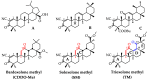
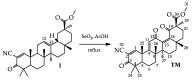
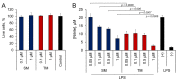
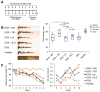
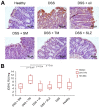
Semi-synthetic triterpenoids, bearing cyano enone functionality in ring A, are considered to be novel promising therapeutic agents with complex inhibitory effects on tissue damage, inflammation and tumor growth. Previously, we showed that the cyano enone-containing 18βH-glycyrrhetinic acid derivative soloxolone methyl (SM) effectively suppressed the inflammatory response of macrophages in vitro and the development of influenza A-induced pneumonia and phlogogen-stimulated paw edema in vivo. In this work, we reported the synthesis of a novel 18βH-glycyrrhetinic acid derivative trioxolone methyl (TM), bearing a 2-cyano-3-oxo-1(2)-en moiety in ring A and a 12,19-dioxo-9(11),13(18)-dien moiety in rings C, D, and E. TM exhibited a high inhibitory effect on nitric oxide (II) production by lipopolysaccharide-stimulated J774 macrophages in vitro and dextran sulfate sodium (DSS)-induced colitis in mice, displaying higher anti-inflammatory activity in comparison with SM. TM effectively suppressed the DSS-induced epithelial damage and inflammatory infiltration of colon tissue, the hyperproduction of colonic neutral mucin and TNFα and increased glutathione synthesis. Our in silico analysis showed that Akt1, STAT3 and dopamine receptor D2 can be considered as mediators of the anti-colitic activity of TM. Our findings provided valuable information for a better understanding of the anti-inflammatory activity of cyano enone-bearing triterpenoids and revealed TM as a promising anti-inflammatory candidate.
Keywords: 18βH-glycyrrhetinic acid; CDDO-Me; colitis; cyano enone; inflammation; molecular docking; molecular targets; soloxolone methyl.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Dual Effect of Soloxolone Methyl on LPS-Induced Inflammation In Vitro and In Vivo.Int J Mol Sci. 2020 Oct 23;21(21):7876. doi: 10.3390/ijms21217876. Int J Mol Sci. 2020. PMID: 33114200 Free PMC article.
-
Cyano Enone-Bearing Triterpenoid Soloxolone Methyl Inhibits Epithelial-Mesenchymal Transition of Human Lung Adenocarcinoma Cells In Vitro and Metastasis of Murine Melanoma In Vivo.Molecules. 2020 Dec 14;25(24):5925. doi: 10.3390/molecules25245925. Molecules. 2020. PMID: 33327637 Free PMC article.
-
[Novel Glycyrrhetinic Acid Derivative Soloxolone Methyl Inhibits the Inflammatory Response and Tumor Growth in vivo].Mol Biol (Mosk). 2018 Mar-Apr;52(2):306-313. doi: 10.7868/S0026898418020143. Mol Biol (Mosk). 2018. PMID: 29695699 Russian.
-
Novel 3'-Substituted-1',2',4'-Oxadiazole Derivatives of 18βH-Glycyrrhetinic Acid and Their O-Acylated Amidoximes: Synthesis and Evaluation of Antitumor and Anti-Inflammatory Potential In Vitro and In Vivo.Int J Mol Sci. 2020 May 15;21(10):3511. doi: 10.3390/ijms21103511. Int J Mol Sci. 2020. PMID: 32429154 Free PMC article.
-
New synthetic triterpenoids: potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress.J Nat Prod. 2011 Mar 25;74(3):537-45. doi: 10.1021/np100826q. Epub 2011 Feb 10. J Nat Prod. 2011. PMID: 21309592 Free PMC article. Review.
Cited by
-
Novel Epoxides of Soloxolone Methyl: An Effect of the Formation of Oxirane Ring and Stereoisomerism on Cytotoxic Profile, Anti-Metastatic and Anti-Inflammatory Activities In Vitro and In Vivo.Int J Mol Sci. 2022 Jun 1;23(11):6214. doi: 10.3390/ijms23116214. Int J Mol Sci. 2022. PMID: 35682893 Free PMC article.
-
Core genes involved in the regulation of acute lung injury and their association with COVID-19 and tumor progression: A bioinformatics and experimental study.PLoS One. 2021 Nov 22;16(11):e0260450. doi: 10.1371/journal.pone.0260450. eCollection 2021. PLoS One. 2021. PMID: 34807957 Free PMC article.
-
Dual Effect of Soloxolone Methyl on LPS-Induced Inflammation In Vitro and In Vivo.Int J Mol Sci. 2020 Oct 23;21(21):7876. doi: 10.3390/ijms21217876. Int J Mol Sci. 2020. PMID: 33114200 Free PMC article.
-
Cyano Enone-Bearing Triterpenoid Soloxolone Methyl Inhibits Epithelial-Mesenchymal Transition of Human Lung Adenocarcinoma Cells In Vitro and Metastasis of Murine Melanoma In Vivo.Molecules. 2020 Dec 14;25(24):5925. doi: 10.3390/molecules25245925. Molecules. 2020. PMID: 33327637 Free PMC article.
-
Grafting of 18β-Glycyrrhetinic Acid and Sialic Acid onto Chitosan to Produce a New Amphipathic Chitosan Derivative: Synthesis, Characterization, and Cytotoxicity.Molecules. 2021 Jan 16;26(2):452. doi: 10.3390/molecules26020452. Molecules. 2021. PMID: 33467083 Free PMC article.
References
-
- Eric A., Gary B., Deborah F., Xin J., Robert K., Jr., Patrick O., Melean V. Compounds Including an Anti-Inflammatory Pharmacore and Methods of Use Background of the Invention. WO/2009/146218. Patent. 2009 Apr 20;
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

