Deboronation-Induced Ratiometric Emission Variations of Terphenyl-Based Closo- o-Carboranyl Compounds: Applications to Fluoride-Sensing
- PMID: 32455846
- PMCID: PMC7287808
- DOI: 10.3390/molecules25102413
Deboronation-Induced Ratiometric Emission Variations of Terphenyl-Based Closo- o-Carboranyl Compounds: Applications to Fluoride-Sensing
Abstract
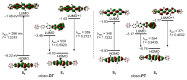
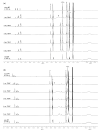
Closo-o-carboranyl compounds bearing the ortho-type perfectly distorted or planar terphenyl rings (closo-DT and closo-PT, respectively) and their nido-derivatives (nido-DT and nido-PT, respectively) were synthesized and fully characterized using multinuclear NMR spectroscopy and elemental analysis. Although the emission spectra of both closo-compounds exhibited intriguing emission patterns in solution at 298 and 77 K, in the film state, closo-DT mainly exhibited a π-π* local excitation (LE)-based emission in the high-energy region, whereas closo-PT produced an intense emission in the low-energy region corresponding to an intramolecular charge transfer (ICT) transition. In particular, the positive solvatochromic effect of closo-PT and theoretical calculation results at the first excited (S1) optimized structure of both closo-compounds strongly suggest that these dual-emissive bands at the high- and low-energy can be assigned to each π-π* LE and ICT transition. Interestingly, both the nido-compounds, nido-DT and nido-PT, exhibited the only LE-based emission in solution at 298 K due to the anionic character of the nido-o-carborane cages, which cannot cause the ICT transitions. The specific emissive features of nido-compounds indicate that the emissive color of closo-PT in solution at 298 K is completely different from that of nido-PT. As a result, the deboronation of closo-PT upon exposure to increasing concentrations of fluoride anion exhibits a dramatic ratiometric color change from orange to deep blue via turn-off of the ICT-based emission. Consequently, the color change response of the luminescence by the alternation of the intrinsic electronic transitions via deboronation as well as the structural feature of terphenyl rings indicates the potential of the developed closo-o-carboranyl compounds that exhibit the intense ICT-based emission, as naked-eye-detectable chemodosimeters for fluoride ion sensing.
Keywords: closo-o-carborane; color change; deboronation; intramolecular charge transfer; nido-o-carborane.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Impact of deboronation on the electronic characteristics of closo-o-carborane: intriguing photophysical changes in triazole-appended carboranyl luminophores.Dalton Trans. 2021 Mar 7;50(9):3207-3215. doi: 10.1039/d0dt04038j. Epub 2021 Feb 12. Dalton Trans. 2021. PMID: 33576753
-
Alteration of intramolecular electronic transition via deboronation of carbazole-based o-carboranyl compound and intriguing 'turn-on' emissive variation.RSC Adv. 2021 Jul 7;11(39):24057-24064. doi: 10.1039/d1ra03716a. eCollection 2021 Jul 6. RSC Adv. 2021. PMID: 35479040 Free PMC article.
-
Tuning the photophysical properties of carboranyl luminophores by closo- to nido-carborane conversion and application to OFF-ON fluoride sensing.Dalton Trans. 2018 Dec 11;47(48):17441-17449. doi: 10.1039/c8dt03771j. Dalton Trans. 2018. PMID: 30488927
-
Fluoride ion complexation and sensing using organoboron compounds.Chem Rev. 2010 Jul 14;110(7):3958-84. doi: 10.1021/cr900401a. Chem Rev. 2010. PMID: 20540560 Review. No abstract available.
-
Redox-active carborane clusters in bond activation chemistry and ligand design.Chem Commun (Camb). 2023 Aug 15;59(66):9918-9928. doi: 10.1039/d3cc03011c. Chem Commun (Camb). 2023. PMID: 37522167 Review.
Cited by
-
Influence of Electronic Environment on the Radiative Efficiency of 9-Phenyl-9H-carbazole-Based ortho-Carboranyl Luminophores.Molecules. 2021 Mar 21;26(6):1763. doi: 10.3390/molecules26061763. Molecules. 2021. PMID: 33801078 Free PMC article.
-
Intramolecular Charge Transfer and Locally Excited State Observation in Push-Pull 1,n‑Bis(4-formylphenoxy)alkanes.ACS Omega. 2025 May 8;10(19):19705-19713. doi: 10.1021/acsomega.5c00766. eCollection 2025 May 20. ACS Omega. 2025. PMID: 40415816 Free PMC article.
-
Celebrating Todd Marder: 65th Birthday and His Contributions to Inorganic Chemistry.Molecules. 2021 Feb 3;26(4):776. doi: 10.3390/molecules26040776. Molecules. 2021. PMID: 33546127 Free PMC article.
References
-
- Bregadze V.I. Dicarba-closo-dodecaboranes C2B10H12 and their derivatives. Chem. Rev. 1992;92:209–223. doi: 10.1021/cr00010a002. - DOI
-
- González-Campo A., Juárez-Pérez E.J., Viñas C., Boury B., Sillanpää R., Kivekäs R., Núñez R. Carboranyl Substituted Siloxanes and Octasilsesquioxanes: Synthesis, Characterization, and Reactivity. Macromolecules. 2008;41:8458–8466. doi: 10.1021/ma801483c. - DOI
-
- Ferrer-Ugalde A., Juárez-Pérez E.J., Teixidor F., Viñas C., Núñez R. Synthesis, Characterization, and Thermal Behavior of Carboranyl–Styrene Decorated Octasilsesquioxanes: Influence of the Carborane Clusters on Photoluminescence. Chem. Eur. J. 2013;19:17021–17030. doi: 10.1002/chem.201302493. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

