Central Modulation of Selective Sphingosine-1-Phosphate Receptor 1 Ameliorates Experimental Multiple Sclerosis
- PMID: 32455907
- PMCID: PMC7291065
- DOI: 10.3390/cells9051290
Central Modulation of Selective Sphingosine-1-Phosphate Receptor 1 Ameliorates Experimental Multiple Sclerosis
Abstract
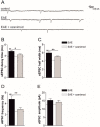
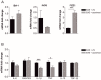
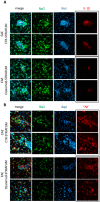
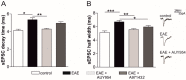
Future treatments of multiple sclerosis (MS), a chronic autoimmune neurodegenerative disease of the central nervous system (CNS), aim for simultaneous early targeting of peripheral immune function and neuroinflammation. Sphingosine-1-phosphate (S1P) receptor modulators are among the most promising drugs with both "immunological" and "non-immunological" actions. Selective S1P receptor modulators have been recently approved for MS and shown clinical efficacy in its mouse model, the experimental autoimmune encephalomyelitis (EAE). Here, we investigated the anti-inflammatory/neuroprotective effects of ozanimod (RPC1063), a S1P1/5 modulator recently approved in the United States for the treatment of MS, by performing ex vivo studies in EAE brain. Electrophysiological experiments, supported by molecular and immunofluorescence analysis, revealed that ozanimod was able to dampen the EAE glutamatergic synaptic alterations, through attenuation of local inflammatory response driven by activated microglia and infiltrating T cells, the main CNS-cellular players of EAE synaptopathy. Electrophysiological studies with selective S1P1 (AUY954) and S1P5 (A971432) agonists suggested that S1P1 modulation is the main driver of the anti-excitotoxic activity mediated by ozanimod. Accordingly, in vivo intra-cerebroventricular treatment of EAE mice with AUY954 ameliorated clinical disability. Altogether these results strengthened the relevance of S1P1 agonists as immunomodulatory and neuroprotective drugs for MS therapy.
Keywords: A971432; AUY954; S1P1; S1P5; T lymphocytes; experimental autoimmune encephalomyelitis (EAE); glutamate synaptic dysfunction; microglia; neuroinflammation; ozanimod; pro-inflammatory cytokines; sphingosine-1-phosphate receptors.
Conflict of interest statement
DC is the recipient of an Institutional grant from Celgene. No personal compensation was received. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results. The other authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

