Spinal Cord Involvement in MS and Other Demyelinating Diseases
- PMID: 32455910
- PMCID: PMC7277673
- DOI: 10.3390/biomedicines8050130
Spinal Cord Involvement in MS and Other Demyelinating Diseases
Abstract
Diagnostic accuracy is poor in demyelinating myelopathies, and therefore a challenge for neurologists in daily practice, mainly because of the multiple underlying pathophysiologic mechanisms involved in each subtype. A systematic diagnostic approach combining data from the clinical setting and presentation with magnetic resonance imaging (MRI) lesion patterns, cerebrospinal fluid (CSF) findings, and autoantibody markers can help to better distinguish between subtypes. In this review, we describe spinal cord involvement, and summarize clinical findings, MRI and diagnostic characteristics, as well as treatment options and prognostic implications in different demyelinating disorders including: multiple sclerosis (MS), neuromyelitis optica spectrum disorder, acute disseminated encephalomyelitis, anti-myelin oligodendrocyte glycoprotein antibody-associated disease, and glial fibrillary acidic protein IgG-associated disease. Thorough understanding of individual case etiology is crucial, not only to provide valuable prognostic information on whether the disorder is likely to relapse, but also to make therapeutic decision-making easier and reduce treatment failures which may lead to new relapses and long-term disability. Identifying patients with monophasic disease who may only require acute management, symptomatic treatment, and subsequent rehabilitation, rather than immunosuppression, is also important.
Keywords: acute disseminated encephalomyelitis; glial fibrillary acidic protein; multiple sclerosis; myelin oligodendrocyte glycoprotein; myelitis; neuromyelitis optica; spinal cord.
Conflict of interest statement
M.M. has nothing to disclose. M.I.G. has received reimbursement for developing educational presentations, from Merck Argentina, Biogen Argentina, Sanofi-Genzyme Argentina, Bayer Inc Argentina and Novartis Argentina, and has received travel/accommodations stipends from Merck Argentina, Biogen Argentina, Roche Argentina, Novartis Argentina, and TEVA Argentina. J.C. is a board member of Merck-Serono Argentina, Biogen-Idec LATAM, Merck-Serono. LATAM, Novartis and Genzyme global. Correale has received reimbursement for developing educational presentations for Merck-Serono Argentina, Merck-Serono LATAM, Biogen-Idec Argentina, Genzyme Argentina, and Novartis Argentina and Roche Argentina as well as professional travel/accommodations stipends. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

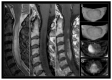
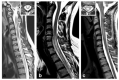
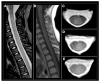
References
Publication types
LinkOut - more resources
Full Text Sources

