From CENTRAL to SENTRAL (SErum aNgiogenesis cenTRAL): Circulating Predictive Biomarkers to Anti-VEGFR Therapy
- PMID: 32456056
- PMCID: PMC7281010
- DOI: 10.3390/cancers12051330
From CENTRAL to SENTRAL (SErum aNgiogenesis cenTRAL): Circulating Predictive Biomarkers to Anti-VEGFR Therapy
Abstract
Background: In the last decade, a series of analyses failed to identify predictive biomarkers of resistance/susceptibility for anti-angiogenic drugs in metastatic colorectal cancer (mCRC). We conducted an exploratory preplanned analysis of serum pro-angiogenic factors (SErum aNgiogenesis-cenTRAL) in 72 mCRC patients enrolled in the phase II CENTRAL (ColorEctalavastiNTRiAlLdh) trial, with the aim to identify potential predictive factors for sensitivity/resistance to first line folinic acid-fluorouracil-irinotecan regimen (FOLFIRI) plus bevacizumab.
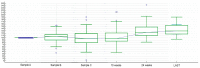
Methods: First-line FOLFIRI/bevacizumab patients were prospectively assessed for the following circulating pro-angiogenic factors, evaluated with ELISA (enzyme-linked immunosorbent assay)-based technique at baseline and at every cycle: Vascular endothelial growth factor A (VEGF-A), hepatocyte growth factor (HGF), stromal derived factor-1 (SDF-1), placental derived growth factor (PlGF), fibroblast growth factor-2 (FGF-2), monocyte chemotactic protein-3 (MCP-3), interleukin-8 (IL-8).
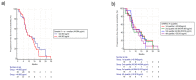
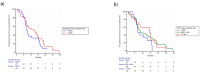
Results: Changes in circulating FGF-2 levels among different blood samples seemed to correlate with clinical outcome. Patients who experienced an increase in FGF-2 levels at the second cycle of chemotherapy compared to baseline, had a median Progression Free Survival (mPFS) of 12.85 vs. 7.57 months (Hazard Ratio-HR: 0.73, 95% Confidence Interval-CI: 0.43-1.27, p = 0.23). Similar results were seen when comparing FGF-2 concentrations between baseline and eight-week time point (mPFS 12.98 vs. 8.00 months, HR: 0.78, 95% CI: 0.46-1.33, p = 0.35).
Conclusions: Our pre-planned, prospective analysis suggests that circulating FGF-2 levels' early increase could be used as a marker to identify patients who are more likely to gain benefit from FOLFIRI/bevacizumab first-line therapy.
Keywords: FGF2; PlGF; VEGF; angiogenesis; bevacizumab; circulating biomarkers; colon cancer.
Conflict of interest statement
The authors declare no conflict of financial or nonfinancial interest.
Figures




References
-
- Oza A.M., Cook A.D., Pfisterer J., Embleton A., Ledermann J.A., Pujade-Lauraine E., Kristensen G., Carey M.S., Beale P., Cervantes A., et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16:928–936. doi: 10.1016/S1470-2045(15)00086-8. - DOI - PMC - PubMed
-
- Yang J.C., Haworth L., Sherry R.M., Hwu P., Schwartzentruber D.J., Topalian S.L., Steinberg S.M., Chen H.X., Rosenberg S.A. A randomized trial of bevacizumab, an anti-vascular endothelial growth. factor antibody, for metastatic renal cancer. N. Engl. J. Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. - DOI - PMC - PubMed
-
- Hurwitz H., Fehrenbacher L., Novotny W., Cartwright T., Hainsworth J., Heim W., Berlin J., Baron A., Griffing S., Holmgren E., et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

