TOM40 Inhibits Ovarian Cancer Cell Growth by Modulating Mitochondrial Function Including Intracellular ATP and ROS Levels
- PMID: 32456076
- PMCID: PMC7281007
- DOI: 10.3390/cancers12051329
TOM40 Inhibits Ovarian Cancer Cell Growth by Modulating Mitochondrial Function Including Intracellular ATP and ROS Levels
Abstract
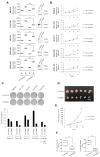
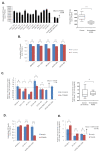
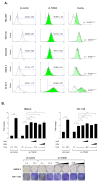
TOM40 is a channel-forming subunit of translocase, which is essential for the movement of proteins into the mitochondria. We found that TOM40 was highly expressed in epithelial ovarian cancer (EOC) cells at both the transcriptional and translational levels; its expression increased significantly during the transformation from normal ovarian epithelial cells to EOC (p < 0.001), and TOM40 expression negatively correlated with disease-free survival (Hazard ratio = 1.79, 95% Confidence inerval 1.16-2.78, p = 0.009). TOM40 knockdown decreased proliferation in several EOC cell lines and reduced tumor burden in an in vivo xenograft mouse model. TOM40 expression positively correlated with intracellular adenosine triphosphate (ATP) levels. The low ATP and high reactive oxygen species (ROS) levels increased the activity of AMP-activated protein kinase (AMPK) in TOM40 knockdown EOC cells. However, AMPK activity did not correlate with declined cell growth in TOM40 knockdown EOC cells. We found that metformin, first-line therapy for type 2 diabetes, effectively inhibited the growth of EOC cell lines in an AMPK-independent manner by inhibiting mitochondria complex I. In conclusion, TOM40 positively correlated with mitochondrial activities, and its association enhances the proliferation of ovarian cancer. Also, metformin is an effective therapeutic option in TOM40 overexpressed ovarian cancer than normal ovarian epithelium.
Keywords: TOM40; epithelial ovarian cancer; metformin; mitochondria.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures





References
-
- Becker L., Bannwarth M., Meisinger C., Hill K., Model K., Krimmer T., Casadio R., Truscott K.N., Schulz G.E., Pfanner N., et al. Preprotein translocase of the outer mitochondrial membrane: Reconstituted Tom40 forms a characteristic TOM pore. J. Mol. Biol. 2005;353:1011–1020. doi: 10.1016/j.jmb.2005.09.019. - DOI - PubMed
LinkOut - more resources
Full Text Sources

