Down-Regulation of miR-23a-3p Mediates Irradiation-Induced Neuronal Apoptosis
- PMID: 32456284
- PMCID: PMC7279507
- DOI: 10.3390/ijms21103695
Down-Regulation of miR-23a-3p Mediates Irradiation-Induced Neuronal Apoptosis
Abstract
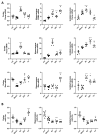
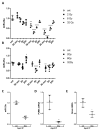
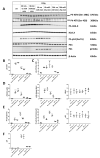
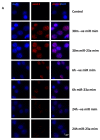
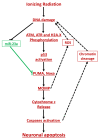
Radiation-induced central nervous system toxicity is a significant risk factor for patients receiving cancer radiotherapy. Surprisingly, the mechanisms responsible for the DNA damage-triggered neuronal cell death following irradiation have yet to be deciphered. Using primary cortical neuronal cultures in vitro, we demonstrated that X-ray exposure induces the mitochondrial pathway of intrinsic apoptosis and that miR-23a-3p plays a significant role in the regulation of this process. Primary cortical neurons exposed to irradiation show the activation of DNA-damage response pathways, including the sequential phosphorylation of ATM kinase, histone H2AX, and p53. This is followed by the p53-dependent up-regulation of the pro-apoptotic Bcl2 family molecules, including the BH3-only molecules PUMA, Noxa, and Bim, leading to mitochondrial outer membrane permeabilization (MOMP) and the release of cytochrome c, which activates caspase-dependent apoptosis. miR-23a-3p, a negative regulator of specific pro-apoptotic Bcl-2 family molecules, is rapidly decreased after neuronal irradiation. By increasing the degradation of PUMA and Noxa mRNAs in the RNA-induced silencing complex (RISC), the administration of the miR-23a-3p mimic inhibits the irradiation-induced up-regulation of Noxa and Puma. These changes result in an attenuation of apoptotic processes such as MOMP, the release of cytochrome c and caspases activation, and a reduction in neuronal cell death. The neuroprotective effects of miR-23a-3p administration may not only involve the direct inhibition of pro-apoptotic Bcl-2 molecules downstream of p53 but also include the attenuation of secondary DNA damage upstream of p53. Importantly, we demonstrated that brain irradiation in vivo results in the down-regulation of miR-23a-3p and the elevation of pro-apoptotic Bcl2-family molecules PUMA, Noxa, and Bax, not only broadly in the cortex and hippocampus, except for Bax, which was up-regulated only in the hippocampus but also selectively in isolated neuronal populations from the irradiated brain. Overall, our data suggest that miR-23a-3p down-regulation contributes to irradiation-induced intrinsic pathways of neuronal apoptosis. These regulated pathways of neurodegeneration may be the target of effective neuroprotective strategies using miR-23a-3p mimics to block their development and increase neuronal survival after irradiation.
Keywords: Bim; MOMP; Noxa; microRNA (miR), Puma; neuronal apoptosis; radiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

