Second-Generation Androgen Receptor Antagonists as Hormonal Therapeutics for Three Forms of Prostate Cancer
- PMID: 32456317
- PMCID: PMC7287767
- DOI: 10.3390/molecules25102448
Second-Generation Androgen Receptor Antagonists as Hormonal Therapeutics for Three Forms of Prostate Cancer
Abstract
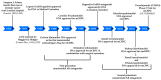
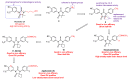
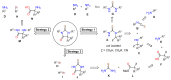
Enzalutamide is the first second-generation nonsteroidal androgen receptor (AR) antagonist with a strong binding affinity to AR. Most significantly, enzalutamide can prolong not only overall survival time and metastatic free survival time for patients with lethal castration-resistant prostate cancer (CRPC), but also castration-resistant free survival time for patients with castration-sensitive prostate cancer (CSPC). Enzalutamide has thus been approved by the US Food and Drug Administration (FDA) for the treatment of both metastatic (in 2012) and non-metastatic (in 2018) CRPC, as well as CSPC (2019). This is an inspiring drug discovery story created by an amazing interdisciplinary collaboration. Equally important, the successful clinical use of enzalutamide proves the notion that the second-generation AR antagonists can serve as hormonal therapeutics for three forms of advanced prostate cancer. This has been further verified by the recent FDA approval of the other two second-generation AR antagonists, apalutamide and darolutamide, for the treatment of prostate cancer. This review focuses on the rational design and discovery of these three second-generation AR antagonists, and then highlights their syntheses, clinical studies, and use. Strategies to overcome the resistance to the second-generation AR antagonists are also reviewed.
Keywords: androgen receptor; apalutamide; darolutamide; enzalutamide; prostate cancer.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
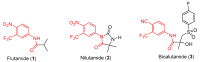

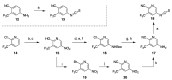
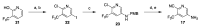

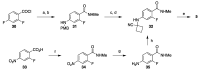
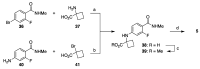
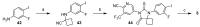
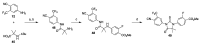

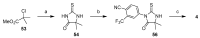
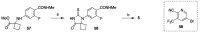
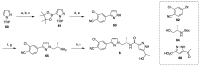


References
-
- Huggins C., Hodges C.V. Prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. - PubMed
-
- Blackard C.E. The Veterans’ Administration Cooperative Urological Research Group studies of carcinoma of the prostate: A review. Cancer Chemother. Rep. 1975;59:225–227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

