Impact of Excited-State Antiaromaticity Relief in a Fundamental Benzene Photoreaction Leading to Substituted Bicyclo[3.1.0]hexenes
- PMID: 32456426
- PMCID: PMC7497645
- DOI: 10.1021/jacs.9b13769
Impact of Excited-State Antiaromaticity Relief in a Fundamental Benzene Photoreaction Leading to Substituted Bicyclo[3.1.0]hexenes
Abstract
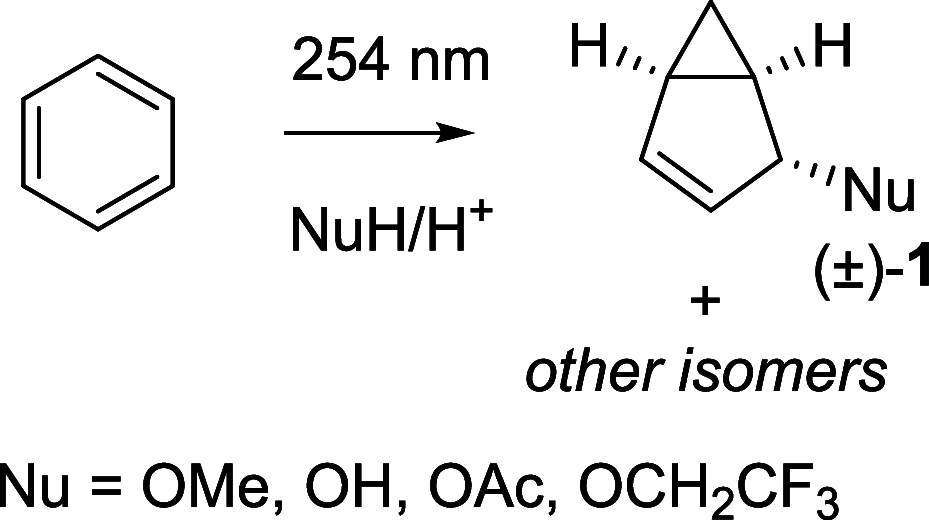
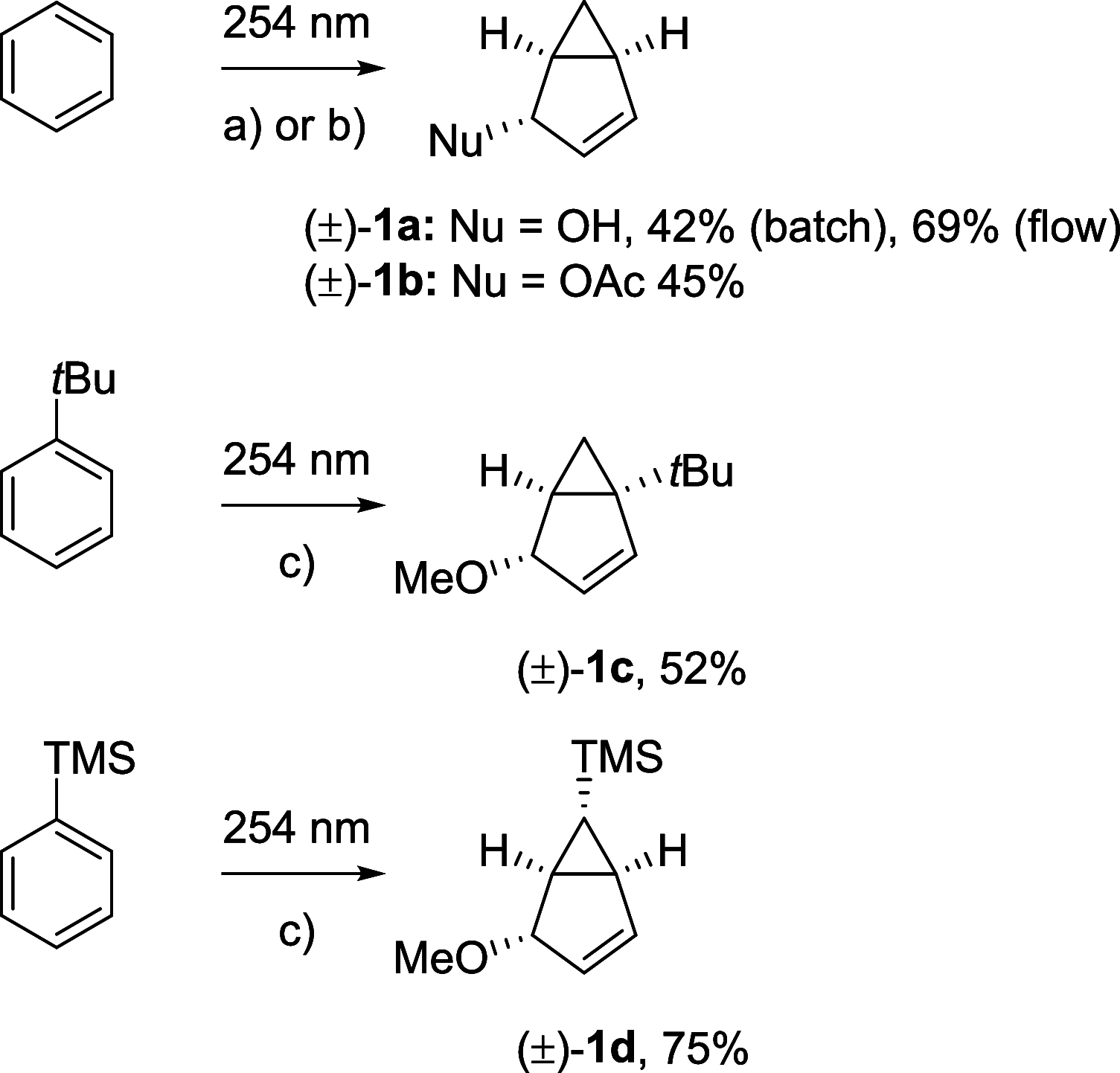
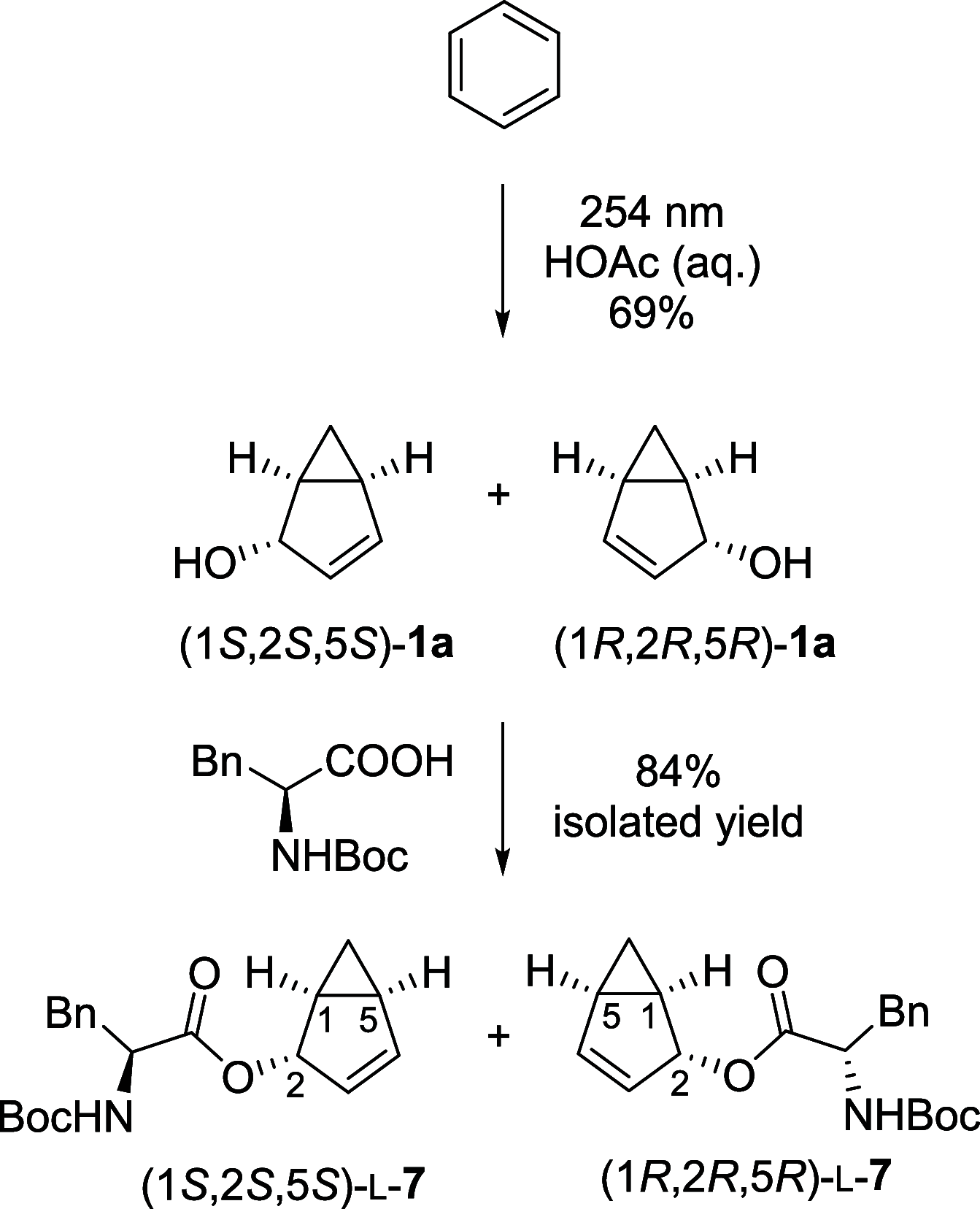
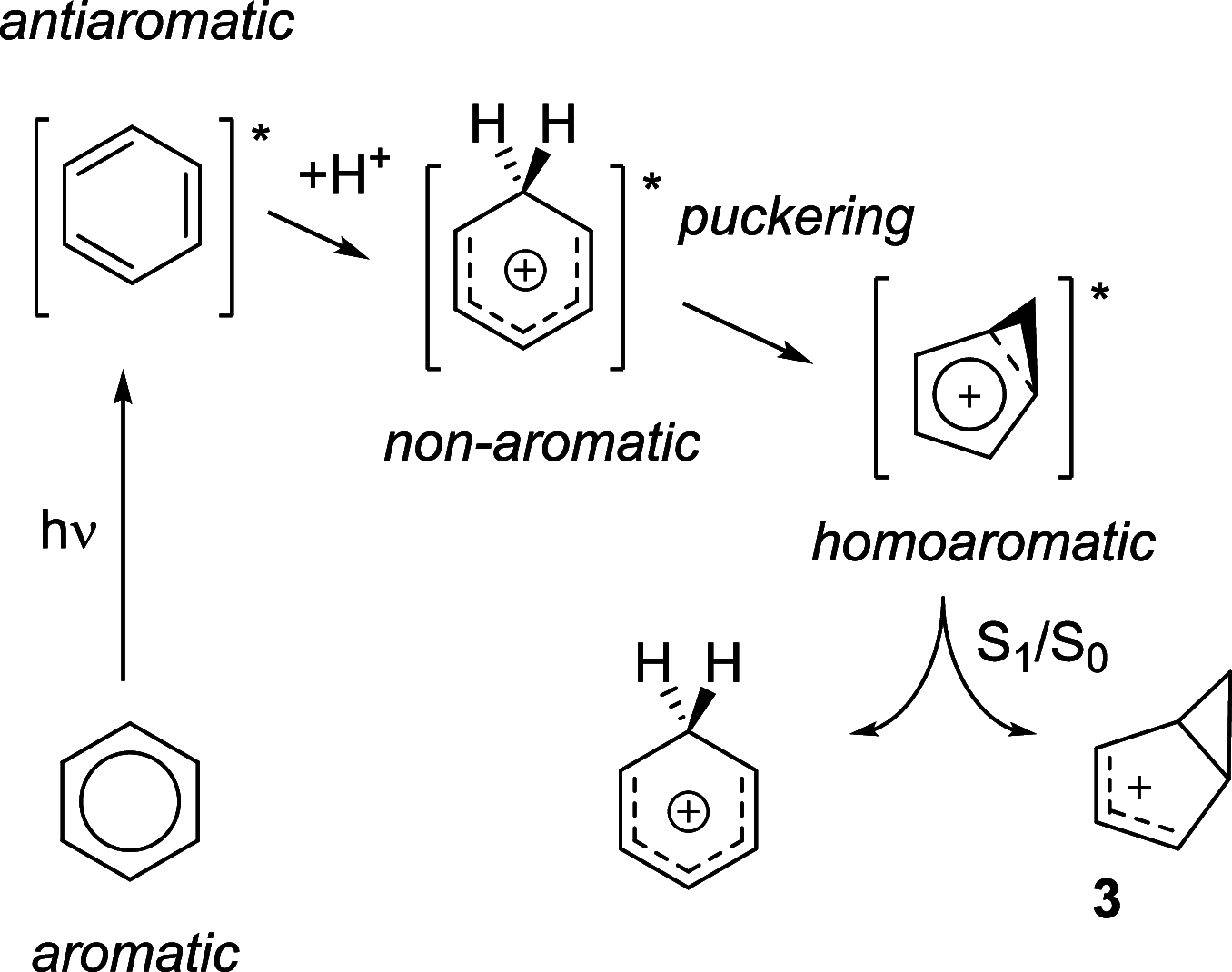
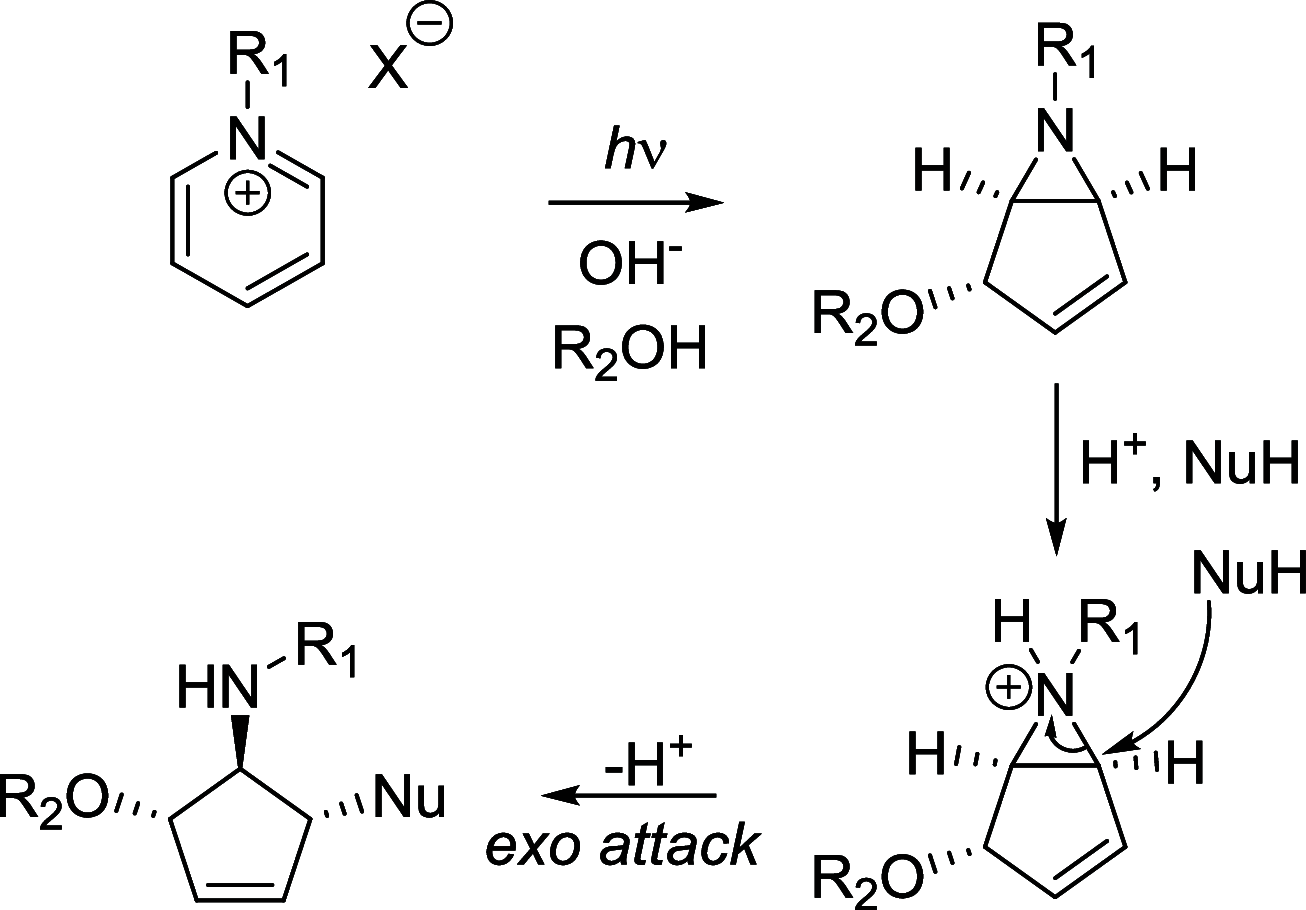
Benzene exhibits a rich photochemistry which can provide access to complex molecular scaffolds that are difficult to access with reactions in the electronic ground state. While benzene is aromatic in its ground state, it is antiaromatic in its lowest ππ* excited states. Herein, we clarify to what extent relief of excited-state antiaromaticity (ESAA) triggers a fundamental benzene photoreaction: the photoinitiated nucleophilic addition of solvent to benzene in acidic media leading to substituted bicyclo[3.1.0]hex-2-enes. The reaction scope was probed experimentally, and it was found that silyl-substituted benzenes provide the most rapid access to bicyclo[3.1.0]hexene derivatives, formed as single isomers with three stereogenic centers in yields up to 75% in one step. Two major mechanism hypotheses, both involving ESAA relief, were explored through quantum chemical calculations and experiments. The first mechanism involves protonation of excited-state benzene and subsequent rearrangement to bicyclo[3.1.0]hexenium cation, trapped by a nucleophile, while the second involves photorearrangement of benzene to benzvalene followed by protonation and nucleophilic addition. Our studies reveal that the second mechanism is operative. We also clarify that similar ESAA relief leads to puckering of S1-state silabenzene and pyridinium ion, where the photorearrangement of the latter is of established synthetic utility. Finally, we identified causes for the limitations of the reaction, information that should be valuable in explorations of similar photoreactions. Taken together, we reveal how the ESAA in benzene and 6π-electron heterocycles trigger photochemical distortions that provide access to complex three-dimensional molecular scaffolds from simple reactants.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- Gleiter R.; Haberhauer G.. Aromaticity and Other Conjugation Effects; Wiley-VCH: Weinheim, Germany, 2012.
-
- Bryce-Smith D.; Gilbert A. The Organic Photochemistry of Benzene—I. Tetrahedron 1976, 32, 1309–1326. 10.1016/0040-4020(76)85002-8. - DOI
-
- Bryce-Smith D.; Gilbert A. The Organic Photochemistry of Benzene—II. Tetrahedron 1977, 33, 2459–2490. 10.1016/0040-4020(77)80069-0. - DOI
Publication types
LinkOut - more resources
Full Text Sources

