Scope, quality, and inclusivity of clinical guidelines produced early in the covid-19 pandemic: rapid review
- PMID: 32457027
- PMCID: PMC7249097
- DOI: 10.1136/bmj.m1936
Scope, quality, and inclusivity of clinical guidelines produced early in the covid-19 pandemic: rapid review
Erratum in
-
Scope, quality, and inclusivity of clinical guidelines produced early in the covid-19 pandemic: rapid review.BMJ. 2020 Jun 12;369:m2371. doi: 10.1136/bmj.m2371. BMJ. 2020. PMID: 32532740 Free PMC article. No abstract available.
Abstract
Objective: To appraise the availability, quality, and inclusivity of clinical guidelines produced in the early stage of the coronavirus disease 2019 (covid-19) pandemic.
Design: Rapid review.
Data sources: Ovid Medline, Ovid Embase, Ovid Global Health, Scopus, Web of Science Core Collection, and WHO Global Index Medicus, searched from inception to 14 Mar 2020. Search strategies applied the CADTH database guidelines search filter, with no limits applied to search results. Further studies were identified through searches of grey literature using the ISARIC network.
Inclusion criteria: Clinical guidelines for the management of covid-19, Middle East respiratory syndrome (MERS), and severe acute respiratory syndrome (SARS) produced by international and national scientific organisations and government and non-governmental organisations relating to global health were included, with no exclusions for language. Regional/hospital guidelines were excluded. Only the earliest version of any guideline was included.
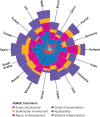
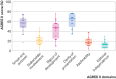
Quality assessment: Quality was assessed using the Appraisal of Guidelines for Research and Evaluation (AGREE) II tool. The quality and contents of early covid-19 guidelines were also compared with recent clinical guidelines for MERS and SARS.
Results: 2836 studies were identified, of which 2794 were excluded after screening. Forty two guidelines were considered eligible for inclusion, with 18 being specific to covid-19. Overall, the clinical guidelines lacked detail and covered a narrow range of topics. Recommendations varied in relation to, for example, the use of antiviral drugs. The overall quality was poor, particularly in the domains of stakeholder involvement, applicability, and editorial independence. Links between evidence and recommendations were limited. Minimal provision was made for vulnerable groups such as pregnant women, children, and older people.
Conclusions: Guidelines available early in the covid-19 pandemic had methodological weaknesses and neglected vulnerable groups such as older people. A framework for development of clinical guidelines during public health emergencies is needed to ensure rigorous methods and the inclusion of vulnerable populations.
Systematic review registration: PROSPERO CRD42020167361.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the Wellcome Trust for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- Norris SL, Sawin VI, Ferri M, Reques Sastre L, Porgo TV. An evaluation of emergency guidelines issued by the World Health Organization in response to four infectious disease outbreaks [correction: PLoS One 2018;13:e0202782]. PLoS One 2018;13:e0198125. 10.1371/journal.pone.0198125 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
