Mouse Retinal Cell Atlas: Molecular Identification of over Sixty Amacrine Cell Types
- PMID: 32457074
- PMCID: PMC7329304
- DOI: 10.1523/JNEUROSCI.0471-20.2020
Mouse Retinal Cell Atlas: Molecular Identification of over Sixty Amacrine Cell Types
Abstract
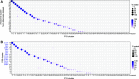
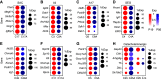
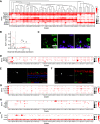
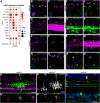
Amacrine cells (ACs) are a diverse class of interneurons that modulate input from photoreceptors to retinal ganglion cells (RGCs), rendering each RGC type selectively sensitive to particular visual features, which are then relayed to the brain. While many AC types have been identified morphologically and physiologically, they have not been comprehensively classified or molecularly characterized. We used high-throughput single-cell RNA sequencing to profile >32,000 ACs from mice of both sexes and applied computational methods to identify 63 AC types. We identified molecular markers for each type and used them to characterize the morphology of multiple types. We show that they include nearly all previously known AC types as well as many that had not been described. Consistent with previous studies, most of the AC types expressed markers for the canonical inhibitory neurotransmitters GABA or glycine, but several expressed neither or both. In addition, many expressed one or more neuropeptides, and two expressed glutamatergic markers. We also explored transcriptomic relationships among AC types and identified transcription factors expressed by individual or multiple closely related types. Noteworthy among these were Meis2 and Tcf4, expressed by most GABAergic and most glycinergic types, respectively. Together, these results provide a foundation for developmental and functional studies of ACs, as well as means for genetically accessing them. Along with previous molecular, physiological, and morphologic analyses, they establish the existence of at least 130 neuronal types and nearly 140 cell types in the mouse retina.SIGNIFICANCE STATEMENT The mouse retina is a leading model for analyzing the development, structure, function, and pathology of neural circuits. A complete molecular atlas of retinal cell types provides an important foundation for these studies. We used high-throughput single-cell RNA sequencing to characterize the most heterogeneous class of retinal interneurons, amacrine cells, identifying 63 distinct types. The atlas includes types identified previously as well as many novel types. We provide evidence for the use of multiple neurotransmitters and neuropeptides, and identify transcription factors expressed by groups of closely related types. Combining these results with those obtained previously, we proposed that the mouse retina contains ∼130 neuronal types and is therefore comparable in complexity to other regions of the brain.
Keywords: GABA; Meis2; RNA-seq; TCF4; glycine; neuropeptide.
Copyright © 2020 the authors.
Figures



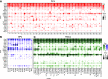
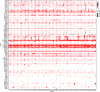

References
-
- Bae JA, Mu S, Kim JS, Turner NL, Tartavull I, Kemnitz N, Jordan CS, Norton AD, Silversmith WM, Prentki R, Sorek M, David C, Jones DL, Bland D, Sterling ALR, Park J, Briggman KL, Seung HS (2018) Digital museum of retinal ganglion cells with dense anatomy and physiology. Cell 173:1293–1306.e19. 10.1016/j.cell.2018.04.040 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
