Collective effects of XMAP215, EB1, CLASP2, and MCAK lead to robust microtubule treadmilling
- PMID: 32457163
- PMCID: PMC7293651
- DOI: 10.1073/pnas.2003191117
Collective effects of XMAP215, EB1, CLASP2, and MCAK lead to robust microtubule treadmilling
Abstract
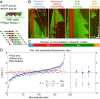
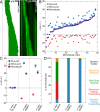
Microtubule network remodeling is essential for fundamental cellular processes including cell division, differentiation, and motility. Microtubules are active biological polymers whose ends stochastically and independently switch between phases of growth and shrinkage. Microtubule treadmilling, in which the microtubule plus end grows while the minus end shrinks, is observed in cells; however, the underlying mechanisms are not known. Here, we use a combination of computational and in vitro reconstitution approaches to determine the conditions leading to robust microtubule treadmilling. We find that microtubules polymerized from tubulin alone can treadmill, albeit with opposite directionality and order-of-magnitude slower rates than observed in cells. We then employ computational simulations to predict that the combinatory effects of four microtubule-associated proteins (MAPs), namely EB1, XMAP215, CLASP2, and MCAK, can promote fast and sustained plus-end-leading treadmilling. Finally, we experimentally confirm the predictions of our computational model using a multi-MAP, in vitro microtubule dynamics assay to reconstitute robust plus-end-leading treadmilling, consistent with observations in cells. Our results demonstrate how microtubule dynamics can be modulated to achieve a dynamic balance between assembly and disassembly at opposite polymer ends, resulting in treadmilling over long periods of time. Overall, we show how the collective effects of multiple components give rise to complex microtubule behavior that may be used for global network remodeling in cells.
Keywords: dynamic instability; in vitro reconstitution; microtubule; microtubule-associated proteins; treadmilling.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Bugyi B., Carlier M. F., Control of actin filament treadmilling in cell motility. Annu. Rev. Biophys. 39, 449–470 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

