The Innate Immune Response to Herpes Simplex Virus 1 Infection Is Dampened in the Newborn Brain and Can Be Modulated by Exogenous Interferon Beta To Improve Survival
- PMID: 32457247
- PMCID: PMC7251210
- DOI: 10.1128/mBio.00921-20
The Innate Immune Response to Herpes Simplex Virus 1 Infection Is Dampened in the Newborn Brain and Can Be Modulated by Exogenous Interferon Beta To Improve Survival
Abstract
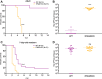
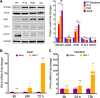
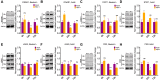
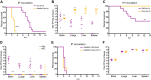
Newborns are particularly susceptible to severe forms of herpes simplex virus 1 (HSV-1) infection, including encephalitis and multisystemic disseminated disease. The underlying age-dependent differences in the immune response that explain this increased susceptibility relative to the adult population remain largely understudied. Using a murine model of HSV-1 infection, we found that newborn mice are largely susceptible to intracranial and intraperitoneal challenge while adult mice are highly resistant. This age-dependent difference correlated with differential basal-level expression of components of innate immune signaling pathways, which resulted in dampened interferon (IFN) signaling in the newborn brain. To explore the possibility of modulating the IFN response in the newborn brain to recapitulate the adult phenotype, we administered exogenous IFN-β in the context of disseminated HSV-1 infection. IFN-β treatment resulted in significantly increased survival and delayed viral neuroinvasion in the newborn. These effects were associated with changes in the type I IFN response in the brain, reduced viral replication in the periphery, and the stabilization of the blood-brain barrier (BBB). Our study reveals important age-dependent differences in the innate immune response to HSV-1 infection and suggests a contribution of the BBB and the brain parenchyma in mediating the increased susceptibility to HSV-1 infection observed in the newborn. These results could provide the basis for potential new therapeutic strategies for life-threatening HSV-1 infection in newborns.IMPORTANCE Herpes simplex virus (HSV) is a ubiquitous human pathogen affecting 50 to 80% of the population in North America and Europe. HSV infection is commonly asymptomatic in the adult population but can result in fatal encephalitis in the newborn. Current treatment with acyclovir has improved mortality in the newborn; however, severe neurologic sequelae are still a major concern following HSV encephalitis. For this reason, there is a critical need to better understand the underlying differences in the immune response between the two age groups that could be used to develop more effective treatments. In this study, we investigated differences in the innate immune response to viral infection in the brains of newborn and adult mice. We found that, similar to humans, newborn mice are more susceptible to HSV infection than the adult. Increased susceptibility was associated with dampened innate immune responses in the newborn brain that could be rescued by administering interferon beta.
Keywords: herpes simplex virus; interferon; newborn; viral encephalitis.
Copyright © 2020 Giraldo et al.
Figures

References
-
- Pebody RG, Andrews N, Brown D, Gopal R, Melker H, François G, Gatcheva N, Hellenbrand W, Jokinen S, Klavs I, Kojouharova M, Kortbeek T, Kriz B, Prosenc K, Roubalova K, Teocharov P, Thierfelder W, Valle M, Damme PV, Vranckx R. 2004. The seroepidemiology of herpes simplex virus type 1 and 2 in Europe. Sex Transm Infect 80:185–191. doi:10.1136/sti.2003.005850. - DOI - PMC - PubMed
-
- Cliffe AR, Arbuckle JH, Vogel JL, Geden MJ, Rothbart SB, Cusack CL, Strahl BD, Kristie TM, Deshmukh M. 2015. Neuronal stress pathway mediating a histone methyl/phospho switch is required for herpes simplex virus reactivation. Cell Host Microbe 18:649–658. doi:10.1016/j.chom.2015.11.007. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

