Field evaluation of a Pan-Lassa rapid diagnostic test during the 2018 Nigerian Lassa fever outbreak
- PMID: 32457420
- PMCID: PMC7250850
- DOI: 10.1038/s41598-020-65736-0
Field evaluation of a Pan-Lassa rapid diagnostic test during the 2018 Nigerian Lassa fever outbreak
Abstract
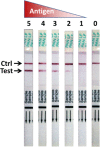
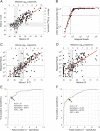
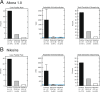
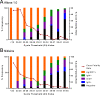
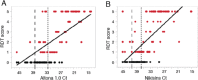
Lassa virus (LASV) is the causative agent of Lassa fever (LF), an often-fatal hemorrhagic disease. LF is endemic in Nigeria, Sierra Leone and other West African countries. Diagnosis of LASV infection is challenged by the genetic diversity of the virus, which is greatest in Nigeria. The ReLASV Pan-Lassa Antigen Rapid Test (Pan-Lassa RDT) is a point-of-care, in vitro diagnostic test that utilizes a mixture of polyclonal antibodies raised against recombinant nucleoproteins of representative strains from the three most prevalent LASV lineages (II, III and IV). We compared the performance of the Pan-LASV RDT to available quantitative PCR (qPCR) assays during the 2018 LF outbreak in Nigeria. For patients with acute LF (RDT positive, IgG/IgM negative) during initial screening, RDT performance was 83.3% sensitivity and 92.8% specificity when compared to composite results of two qPCR assays. 100% of samples that gave Ct values below 22 on both qPCR assays were positive on the Pan-Lassa RDT. There were significantly elevated case fatality rates and elevated liver transaminase levels in subjects whose samples were RDT positive compared to RDT negative.
Conflict of interest statement
MLB, DSG, JSS, PCS, CTH, LMB and RFG Are members of the The Viral Hemorrhagic Fever Consortium (
Figures


References
-
- WHO. Lassa Fever – Benin, Togo and Burkina Faso. Disease outbreak news 10 March 2017 (2017).
-
- ECDC. Lassa fever in Nigeria, Benin, Togo, Germany and USA. European Centre for Disease Prevention and Control 23 March 2016 (2016).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

