Revisiting the role of CD4+ T cells in cancer immunotherapy-new insights into old paradigms
- PMID: 32457487
- PMCID: PMC7886651
- DOI: 10.1038/s41417-020-0183-x
Revisiting the role of CD4+ T cells in cancer immunotherapy-new insights into old paradigms
Abstract
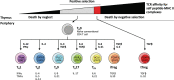
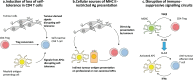
Cancer immunotherapy has revolutionised cancer treatment, with immune checkpoint blockade (ICB) therapy and adoptive cell therapy (ACT) increasingly becoming standard of care across a growing number of cancer indications. While the majority of cancer immunotherapies focus on harnessing the anti-tumour CD8+ cytotoxic T cell response, the potential role of CD4+ 'helper' T cells has largely remained in the background. In this review, we give an overview of the multifaceted role of CD4+ T cells in the anti-tumour immune response, with an emphasis on recent evidence that CD4+ T cells play a bigger role than previously thought. We illustrate their direct anti-tumour potency and their role in directing a sustained immune response against tumours. We further highlight the emerging observation that CD4+ T cell responses against tumours tend to be against self-derived epitopes. These recent trends raise vital questions and considerations that will profoundly affect the rational design of immunotherapies to leverage on the full potential of the immune system against cancer.
Conflict of interest statement
H.C.T. is the Chief Medical Officer of Tessa Therapeutics Limited.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

