Kawasaki disease: pathophysiology and insights from mouse models
- PMID: 32457494
- PMCID: PMC7250272
- DOI: 10.1038/s41584-020-0426-0
Kawasaki disease: pathophysiology and insights from mouse models
Abstract
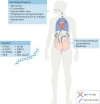
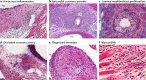
Kawasaki disease is an acute febrile illness and systemic vasculitis of unknown aetiology that predominantly afflicts young children, causes coronary artery aneurysms and can result in long-term cardiovascular sequelae. Kawasaki disease is the leading cause of acquired heart disease among children in the USA. Coronary artery aneurysms develop in some untreated children with Kawasaki disease, leading to ischaemic heart disease and myocardial infarction. Although intravenous immunoglobulin (IVIG) treatment reduces the risk of development of coronary artery aneurysms, some children have IVIG-resistant Kawasaki disease and are at increased risk of developing coronary artery damage. In addition, the lack of specific diagnostic tests and biomarkers for Kawasaki disease make early diagnosis and treatment challenging. The use of experimental mouse models of Kawasaki disease vasculitis has considerably improved our understanding of the pathology of the disease and helped characterize the cellular and molecular immune mechanisms contributing to cardiovascular complications, in turn leading to the development of innovative therapeutic approaches. Here, we outline the pathophysiology of Kawasaki disease and summarize and discuss the progress gained from experimental mouse models and their potential therapeutic translation to human disease.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54:271–276. - PubMed
-
- Newburger JW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747–2771. doi: 10.1161/01.CIR.0000145143.19711.78. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

