RNA delivery by extracellular vesicles in mammalian cells and its applications
- PMID: 32457507
- PMCID: PMC7249041
- DOI: 10.1038/s41580-020-0251-y
RNA delivery by extracellular vesicles in mammalian cells and its applications
Abstract
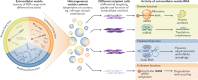
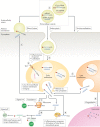
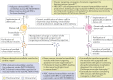
The term 'extracellular vesicles' refers to a heterogeneous population of vesicular bodies of cellular origin that derive either from the endosomal compartment (exosomes) or as a result of shedding from the plasma membrane (microvesicles, oncosomes and apoptotic bodies). Extracellular vesicles carry a variety of cargo, including RNAs, proteins, lipids and DNA, which can be taken up by other cells, both in the direct vicinity of the source cell and at distant sites in the body via biofluids, and elicit a variety of phenotypic responses. Owing to their unique biology and roles in cell-cell communication, extracellular vesicles have attracted strong interest, which is further enhanced by their potential clinical utility. Because extracellular vesicles derive their cargo from the contents of the cells that produce them, they are attractive sources of biomarkers for a variety of diseases. Furthermore, studies demonstrating phenotypic effects of specific extracellular vesicle-associated cargo on target cells have stoked interest in extracellular vesicles as therapeutic vehicles. There is particularly strong evidence that the RNA cargo of extracellular vesicles can alter recipient cell gene expression and function. During the past decade, extracellular vesicles and their RNA cargo have become better defined, but many aspects of extracellular vesicle biology remain to be elucidated. These include selective cargo loading resulting in substantial differences between the composition of extracellular vesicles and source cells; heterogeneity in extracellular vesicle size and composition; and undefined mechanisms for the uptake of extracellular vesicles into recipient cells and the fates of their cargo. Further progress in unravelling the basic mechanisms of extracellular vesicle biogenesis, transport, and cargo delivery and function is needed for successful clinical implementation. This Review focuses on the current state of knowledge pertaining to packaging, transport and function of RNAs in extracellular vesicles and outlines the progress made thus far towards their clinical applications.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

