Sleep Disturbances in MCI and AD: Neuroinflammation as a Possible Mediating Pathway
- PMID: 32457592
- PMCID: PMC7227443
- DOI: 10.3389/fnagi.2020.00069
Sleep Disturbances in MCI and AD: Neuroinflammation as a Possible Mediating Pathway
Abstract
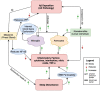
Mild cognitive impairment (MCI) and Alzheimer's disease (AD) affect a high proportion of the elderly population with an increasing prevalence. Sleep disturbances are frequent in those with MCI and AD. This review summarizes existing research on sleep disturbances and neuroinflammation in MCI and AD. Although strong evidence supports various pathways linking sleep and AD pathology, the temporal direction of this central relationship is not yet known. Improved understanding of sleep disturbance and neuroinflammation in MCI and AD may aid in the identification of targets for their prevention.
Keywords: Alzheimer’s disease; aging; mild cognitive impairment; neuroinflammation; sleep.
Copyright © 2020 Pak, Onen, Bliwise, Kutner, Russell and Onen.
Figures
References
-
- Blackwell T., Yaffe K., Ancoli-Israel S., Schneider J. L., Cauley J. A., Hillier T. A., et al. (2006). Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 61 405–410. 10.1093/gerona/61.4.405 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

