Clinical and Imaging Markers of Prodromal Parkinson's Disease
- PMID: 32457695
- PMCID: PMC7225301
- DOI: 10.3389/fneur.2020.00395
Clinical and Imaging Markers of Prodromal Parkinson's Disease
Abstract
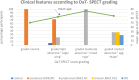
The diagnosis of Parkinson's disease (PD) relies on the clinical effects of dopamine deficiency, including bradykinesia, rigidity and tremor, usually manifesting asymmetrically. Misdiagnosis is common, due to overlap of symptoms with other neurodegenerative disorders such as multiple system atrophy and progressive supranuclear palsy, and only autopsy can definitively confirm the disease. Motor deficits generally appear when 50-60% of dopaminergic neurons in the substantia nigra are already lost, limiting the effectiveness of potential neuroprotective therapies. Today, we consider PD to be not just a movement disorder, but rather a complex syndrome non-motor symptoms (NMS) including disorders of sleep-wake cycle regulation, cognitive impairment, disorders of mood and affect, autonomic dysfunction, sensory symptoms and pain. Symptomatic LRRK2 mutation carriers share non-motor features with individuals with sporadic PD, including hyposmia, constipation, impaired color discrimination, depression, and sleep disturbance. Following the assumption that the pre-symptomatic gene mutation carriers will eventually exhibit clinical symptoms, their neuroimaging results can be extended to the pre-symptomatic stage of PD. The long latent phase of PD, termed prodromal-PD, represents an opportunity for early recognition of incipient PD. Early recognition could allow initiation of possible neuroprotective therapies at a stage when therapies might be most effective. The number of markers with the sufficient level of evidence to be included in the MDS research criteria for prodromal PD have increased during the last 10 years. Here, we review the approach to prodromal PD, with an emphasis on clinical and imaging markers and report results from our neuroimaging study, a retrospective evaluation of a cohort of 39 participants who underwent DAT-SPECT scan as part of their follow up. The study was carried out to see if it was possible to detect subclinical signs in the preclinical (neurodegenerative processes have commenced, but there are no evident symptoms or signs) and prodromal (symptoms and signs are present, but are yet insufficient to define disease) stages of PD.
Keywords: DAT-SPECT; LRRK2; Parkinson's disease; olfaction; prodromal markers.
Copyright © 2020 Hustad and Aasly.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

