From Normal Flora to Brain Abscesses: A Review of Streptococcus intermedius
- PMID: 32457718
- PMCID: PMC7221147
- DOI: 10.3389/fmicb.2020.00826
From Normal Flora to Brain Abscesses: A Review of Streptococcus intermedius
Abstract
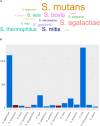
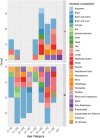
Streptococcus intermedius is a β-hemolytic Gram-positive member of the Streptococcus anginosus group (SAG). Despite being a part of the normal microbiota, it is one of the most common pathogens associated with brain and liver abscesses and thoracic empyema, increasing as a result the morbidity and mortality rates in affected patients. Though there are numerous published case reports on S. intermedius infections, it is still understudied compared to other SAG members. Our knowledge of the genomic factors contributing to its dissemination to the brain and abscess development is also limited to few characterized genes. In this review, we summarize our current knowledge on S. intermedius identification methods, virulence factors, and insight provided by the whole-genome and correlate patients' metadata, symptoms, and disease outcome with S. intermedius infections in 101 recent case reports obtained from PubMed. This combined information highlights the gaps in our understanding of S. intermedius pathogenesis, suggesting future research directions to unveil the factors contributing to abscess development.
Keywords: Streptococcus anginosus group (SAG); Streptococcus intermedius; brain abscess; fibronectin (FN); laminin; virulence.
Copyright © 2020 Issa, Salloum and Tokajian.
Figures


References
-
- Anderton J. M., Rajam G., Romero-Steiner S., Summer S., Kowalczyk A. P., Carlone G. M., et al. (2007). E-cadherin is a receptor for the common protein pneumococcal surface adhesin A (PsaA) of Streptococcus pneumoniae. Microb. Pathog. 42 225–236. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

