Minimally Invasive Surfactant Administration for the Treatment of Neonatal Respiratory Distress Syndrome: A Multicenter Randomized Study in China
- PMID: 32457854
- PMCID: PMC7221055
- DOI: 10.3389/fped.2020.00182
Minimally Invasive Surfactant Administration for the Treatment of Neonatal Respiratory Distress Syndrome: A Multicenter Randomized Study in China
Abstract
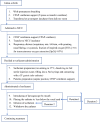
Background/Aims: Nasal continuous positive airway pressure (nCPAP) was recommended as the initial respiratory support for spontaneous breathing in infants with very low birth weight and neonatal respiratory distress syndrome (NRDS). Less invasive surfactant administration (LISA) and minimally invasive surfactant therapy (MIST) have been reported to reduce the incidence of bronchopulmonary dysplasia (BPD). This study aimed to explore the applicability of minimally invasive surfactant administration (MISA) in China. Materials and Methods: MISA was a randomized controlled study conducted at eight level III neonatal intensive care units (NICUs) in China. Spontaneously breathing infants born at 25+0 to 31+6 weeks' gestation who progressively developed respiratory distress during the first 6 h after birth were randomly assigned to receive MISA or endotracheal intubation surfactant administration (EISA). The primary outcome was the difference in the morbidity of BPD between two groups of infants with MISA and EISA at 36 weeks corrected gestational age. Results: Demographic and clinical characteristics of the 151 infants in the MISA group were similar to the 147 infants in the EISA group. The comparison showed no clear benefits in the MISA group in the incidence of BPD, while infants from the EISA group had higher rates of patent ductus arteriosus (PDA) (60.5 vs. 41.1%, p = 0.001). The duration of surfactant infusion and the total time of surfactant administration in the MISA group were significantly longer than in the EISA group. A slightly increased heart rate was noted 1 h post surfactant administration in the EISA group. In subgroup analysis, the comparison of 51 smaller (<30 weeks) preterm infants, named MISAs (n = 31) and EISAs (n = 20), showed a significant reduction of BPD (29.0 vs. 70.0%, p = 0.004) and PDA (29.0 vs. 65.0%, p = 0.011). In the subgroup analysis of blood gas, arterial oxygen saturation (SaO2) value at 1 and 12 h and partial pressure of arterial oxygen (PaO2) at 12 h were all higher in the EISA group compared to the MISA group. Conclusion: MISA had no clear benefit on the incidence of BPD, but it was related to a reduction in PDA. It is an appropriate therapy for spontaneous breathing in infants with extremely low birth weight and NRDS.
Keywords: bronchopulmonary dysplasia; extremely low birth weight infants; minimally invasive surfactant administration; neonatal respiratory distress syndrome; patent ductus arteriosus; preterm infants.
Copyright © 2020 Han, Liu, Zhang, Guo, Zhang, Duan, Sun, Liu, Zhang, Zhang, Liu, Bao, Xiao, Liu, Jiang, Zheng, Tian, Gao, Zhang, Guo, Li and Tong.
Figures
References
-
- Avery ME, Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. Am J Dis Child. (1959) 97:517–23. - PubMed
LinkOut - more resources
Full Text Sources