Molecular Mechanisms of Adipogenesis: The Anti-adipogenic Role of AMP-Activated Protein Kinase
- PMID: 32457917
- PMCID: PMC7226927
- DOI: 10.3389/fmolb.2020.00076
Molecular Mechanisms of Adipogenesis: The Anti-adipogenic Role of AMP-Activated Protein Kinase
Abstract
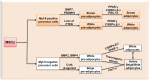
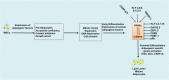
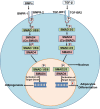
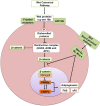
Obesity is now a widespread disorder, and its prevalence has become a critical concern worldwide, due to its association with common co-morbidities like cancer, cardiovascular diseases and diabetes. Adipose tissue is an endocrine organ and therefore plays a critical role in the survival of an individual, but its dysfunction or excess is directly linked to obesity. The journey from multipotent mesenchymal stem cells to the formation of mature adipocytes is a well-orchestrated program which requires the expression of several genes, their transcriptional factors, and signaling intermediates from numerous pathways. Understanding all the intricacies of adipogenesis is vital if we are to counter the current epidemic of obesity because the limited understanding of these intricacies is the main barrier to the development of potent therapeutic strategies against obesity. In particular, AMP-Activated Protein Kinase (AMPK) plays a crucial role in regulating adipogenesis - it is arguably the central cellular energy regulation protein of the body. Since AMPK promotes the development of brown adipose tissue over that of white adipose tissue, special attention has been given to its role in adipose tissue development in recent years. In this review, we describe the molecular mechanisms involved in adipogenesis, the role of signaling pathways and the substantial role of activated AMPK in the inhibition of adiposity, concluding with observations which will support the development of novel chemotherapies against obesity epidemics.
Keywords: AMPK; BAT; WAT; adipogenesis; beige/brite adipocytes; obesity.
Copyright © 2020 Ahmad, Serpell, Fong and Wong.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

