Potential new treatment strategies for COVID-19: is there a role for bromhexine as add-on therapy?
- PMID: 32458206
- PMCID: PMC7249615
- DOI: 10.1007/s11739-020-02383-3
Potential new treatment strategies for COVID-19: is there a role for bromhexine as add-on therapy?
Abstract
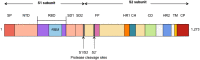
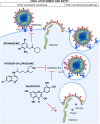
Of huge importance now is to provide a fast, cost-effective, safe, and immediately available pharmaceutical solution to curb the rapid global spread of SARS-CoV-2. Recent publications on SARS-CoV-2 have brought attention to the possible benefit of chloroquine in the treatment of patients infected by SARS-CoV-2. Whether chloroquine can treat SARS-CoV-2 alone and also work as a prophylactic is doubtful. An effective prophylactic medication to prevent viral entry has to contain, at least, either a protease inhibitor or a competitive virus ACE2-binding inhibitor. Using bromhexine at a dosage that selectively inhibits TMPRSS2 and, in so doing, inhibits TMPRSS2-specific viral entry is likely to be effective against SARS-CoV-2. We propose the use of bromhexine as a prophylactic and treatment. We encourage the scientific community to assess bromhexine clinically as a prophylactic and curative treatment. If proven to be effective, this would allow a rapid, accessible, and cost-effective application worldwide.
Keywords: Bromhexine; COVID-19; Prophylactic; Protease inhibitor; SARS-CoV-2; Treatment.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- World Health Organization (2020) Coronavirus disease (COVID-2019) press briefings. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/media-re.... Accessed 22 Mar 2020
-
- World Health Organization (2020) Coronavirus Disease (COVID-2019) Situation Reports 1–89. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio.... Accessed 18 Apr 2020
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

