Acromegaly, inflammation and cardiovascular disease: a review
- PMID: 32458292
- PMCID: PMC7560935
- DOI: 10.1007/s11154-020-09560-x
Acromegaly, inflammation and cardiovascular disease: a review
Abstract
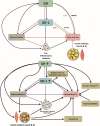
Acromegaly is characterized by Growth Hormone (GH) and Insulin-like Growth Factor 1 (IGF-1) excess. Uncontrolled acromegaly is associated with a strongly increased risk of cardiovascular disease (CVD), and numerous cardiovascular risk factors remain present after remission. GH and IGF-1 have numerous effects on the immune and cardiovascular system. Since endothelial damage and systemic inflammation are strongly linked to the development of CVD, and have been suggested to be present in both controlled as uncontrolled acromegaly, they may explain the presence of both micro- and macrovascular dysfunction in these patients. In addition, these changes seem to be only partially reversible after remission, as illustrated by the often reported presence of endothelial dysfunction and microvascular damage in controlled acromegaly. Previous studies suggest that insulin resistance, oxidative stress, and endothelial dysfunction are involved in the development of CVD in acromegaly. Not surprisingly, these processes are associated with systemic inflammation and respond to GH/IGF-1 normalizing treatment.
Keywords: Acromegaly; Cardiovascular disease; Cytokines; Growth hormone; Inflammation; Insulin-like growth Factor-1.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

