A Diagnostic Loop-Mediated Isothermal Amplification Method to Distinguish Helicoverpa armigera (Lepidoptera: Noctuidae) From Other Related Species in the New World
- PMID: 32458991
- PMCID: PMC7251530
- DOI: 10.1093/jisesa/ieaa046
A Diagnostic Loop-Mediated Isothermal Amplification Method to Distinguish Helicoverpa armigera (Lepidoptera: Noctuidae) From Other Related Species in the New World
Abstract
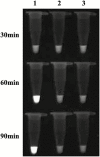
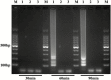
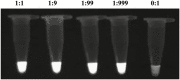
Helicoverpa armigera (Hübner) is a notorious agricultural pest native to the Old World. Recently, its invasion into South and Central America has become a serious problem in the New World. The rapid detection of invasive pests is essential to eradicate them and prevent establishment. However, an extremely similar species, H. zea (Boddie) distributed in the New World makes identification difficult. Helicoverpa armigera and H. zea have only minor differences in male genitalia to separate them morphologically. Both species are attracted to the same pheromone lure, and it takes considerable time and effort to identify them from bulk samples obtained during trap monitoring. Although several molecular approaches based on PCR have been reported, these methods require expensive equipment and are unsuitable for onsite diagnostics. Here, we developed a rapid and convenient diagnostic method based on the loop-mediated isothermal amplification to distinguish H. armigera from related species: H. zea, H. assulta (Guenée), H. punctigera (Wallengren), and Chloridea virescens (Fabricius). The diagnostic method makes it possible to detect H. armigera within 90 min only using simple equipment. The method also worked with mixed DNA templates containing excess DNA from H. zea at the ratio of 1:999 (H. armigera:H. zea). This method can be an effective tool for onsite diagnostics during monitoring surveys for invasive H. armigera.
Keywords: Helicoverpa; loop-mediated isothermal amplification; molecular identification.
© The Author(s) 2020. Published by Oxford University Press on behalf of Entomological Society of America.
Figures



References
-
- Behere G. T., Tay W. T., Russell D. A., and Batterham P.. . 2008. Molecular markers to discriminate among four pest species of Helicoverpa (Lepidoptera: Noctuidae). Bull. Entomol. Res. 98: 599–603. - PubMed
-
- Blaser S., Diem H., von Felten A., Gueuning M., Andreou M., Boonham N., Tomlinson J., Müller P., Utzinger J., Frey J. E., . et al. 2018. From laboratory to point of entry: development and implementation of a loop-mediated isothermal amplification (LAMP)-based genetic identification system to prevent introduction of quarantine insect species. Pest Manag. Sci. 74: 1504–1512. - PMC - PubMed
-
- Bueno A. de F., and Sosa-Gómez D. R.. . 2014. The old world bollworm in the neotropical region: the experience of Brazilian growers with Helicoverpa armigera. Outlooks Pest Manag. 25: 261–264.
-
- Castiglioni E., Perini Clérison R., Chiaravalle W., Arnemann Jonas A., Ugalde G., and Guedes Jerson V. C.. . 2016. Primer registro de ocurrencia de Helicoverpa armigera (Hübner, 1808) (Lepidoptera: Noctuidae) en soja, en Uruguay. Agrocienc. Sitio Repar. 20: 31–35.
-
- Cunningham J. P., and Zalucki M. P.. . 2014. Understanding heliothine (Lepidoptera: Heliothinae) pests: what is a host plant? J. Econ. Entomol. 107: 881–896. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

