Wrapping Up Hydrophobic Hydration: Locality Matters
- PMID: 32459100
- PMCID: PMC8253475
- DOI: 10.1021/acs.jpclett.0c00846
Wrapping Up Hydrophobic Hydration: Locality Matters
Abstract
Water, being the universal solvent, acts as a competing agent in fundamental processes, such as folding, aggregation or biomolecular recognition. A molecular understanding of hydrophobic hydration is of central importance to understanding the subtle free energy differences, which dictate function. Ab initio and classical molecular dynamics simulations yield two distinct hydration water populations in the hydration shell of solvated tert-butanol noted as "HB-wrap" and "HB-hydration2bulk". The experimentally observed hydration water spectrum can be dissected into two modes, centered at 164 and 195 cm-1. By comparison to the simulations, these two bands are attributed to the "HB-wrap" and "HB-hydration2bulk" populations, respectively. We derive a quantitative correlation between the population in each of these two local water coordination motifs and the temperature dependence of the solvation entropy. The crossover from entropy to enthalpy dominated solvation at elevated temperatures, as predicted by theory and observed experimentally, can be rationalized in terms of the distinct temperature stability and thermodynamic signatures of "HB-wrap" and "HB-hydration2bulk".
Conflict of interest statement
The authors declare no competing financial interest.
Figures
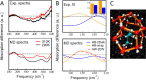
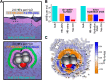
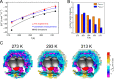
 with Tref =
400 K (see the
with Tref =
400 K (see the References
-
- Frank F. S.; Evans M. W. Free volume and entropy in condensed systems III. Entropy in binary liquid mixtures; Partial molar entropy in dilute solutions; Structure and thermodynamics in aqueous electrolytes. J. Chem. Phys. 1945, 13, 507–532. 10.1063/1.1723985. - DOI
-
- Lum K.; Chandler D.; Weeks J. D. Hydrophobicity at small and large length scales. J. Phys. Chem. B 1999, 103, 4570–4577. 10.1021/jp984327m. - DOI
LinkOut - more resources
Full Text Sources

