Tailored Medication Adherence Incentives Using mHealth for Children With High-Risk Asthma (TAICAM): Protocol for a Randomized Controlled Trial
- PMID: 32459653
- PMCID: PMC7459431
- DOI: 10.2196/16711
Tailored Medication Adherence Incentives Using mHealth for Children With High-Risk Asthma (TAICAM): Protocol for a Randomized Controlled Trial
Abstract
Background: Poor adherence to inhaled corticosteroid medications for children with high-risk asthma is both well documented and poorly understood. It has a disproportionate prevalence and impact on children of minority demographics in urban settings. Financial incentives have been shown to be a compelling method to engage those in a high-risk asthma population, but whether adherence can be maintained by offering financial incentives and how these incentives can be used to sustain high adherence are unknown.
Objective: The aim of this study is to determine the marginal effects of a financial incentive-based intervention on inhaled corticosteroid adherence, health care system use, and costs.
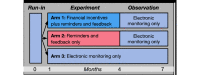
Methods: Participants include children aged 5 to 12 years who have had either at least two hospitalizations or one hospitalization and one emergency department visit for asthma in the year prior to their enrollment (and their caregivers). Participants are given an electronic inhaler sensor in order to track their medication use over a period of 7 months. After a 1-month period of observation, participants are randomized to 1 of 3 arms for a 3-month period. Participants in arm 1 receive daily text message reminders, feedback, and gain-framed, nominal financial incentives; participants in arm 2 receive daily text message reminders and feedback only, and participants in arm 3 receive no reminders, feedback, or incentives. All participants are subsequently observed for an additional 3-month period with no reminders, feedback, or incentives to assess whether any sustained effects are apparent.
Results: Study enrollment began in September 2019 with a target sample size of N=125 children. As of June 2020, 61 children have been enrolled. Data collection is estimated to be completed in June 2022, and analyses will be completed by June 2023.
Conclusions: This study will provide data that will help to determine whether a financial incentive-based mobile health intervention for promoting inhaled corticosteroid use can be effective in patients with high-risk asthma over longer periods.
Trial registration: Clinicaltrial.gov NCT03907410; https://clinicaltrials.gov/ct2/show/NCT03907410.
International registered report identifier (irrid): DERR1-10.2196/16711.
Keywords: adolescent; asthma; behavior change; child; clinical protocols; financial incentives; medication adherence; metered dose inhalers; minority; pediatrics; randomized controlled trial; text messaging.
©Brittney R Henderson, Carina M Flaherty, G Chandler Floyd, Jack You, Rui Xiao, Tyra C Bryant-Stephens, Victoria A Miller, Chris Feudtner, Chén Collin Kenyon. Originally published in JMIR Research Protocols (http://www.researchprotocols.org), 17.08.2020.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- Farber HJ, Johnson C, Beckerman RC. Young inner-city children visiting the emergency room (ER) for asthma: risk factors and chronic care behaviors. J Asthma. 1998;35(7):547–52. - PubMed
-
- Rand CS, Butz AM, Kolodner K, Huss K, Eggleston P, Malveaux F. Emergency department visits by urban African American children with asthma. J Allergy Clin Immunol. 2000 Jan;105(1 Pt 1):83–90. - PubMed
-
- Bartlett SJ, Lukk P, Butz A, Lampros-Klein F, Rand CS. Enhancing medication adherence among inner-city children with asthma: results from pilot studies. J Asthma. 2002 Feb;39(1):47–54. - PubMed
-
- McNally KA, Rohan J, Schluchter M, Riekert KA, Vavrek P, Schmidt A, Redline S, Kercsmar C, Drotar D. Adherence to combined montelukast and fluticasone treatment in economically disadvantaged african american youth with asthma. J Asthma. 2009 Nov;46(9):921–7. doi: 10.3109/02770900903229651. - DOI - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials