Remdesivir for 5 or 10 Days in Patients with Severe Covid-19
- PMID: 32459919
- PMCID: PMC7377062
- DOI: 10.1056/NEJMoa2015301
Remdesivir for 5 or 10 Days in Patients with Severe Covid-19
Abstract
Background: Remdesivir is an RNA polymerase inhibitor with potent antiviral activity in vitro and efficacy in animal models of coronavirus disease 2019 (Covid-19).
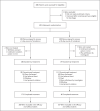
Methods: We conducted a randomized, open-label, phase 3 trial involving hospitalized patients with confirmed SARS-CoV-2 infection, oxygen saturation of 94% or less while they were breathing ambient air, and radiologic evidence of pneumonia. Patients were randomly assigned in a 1:1 ratio to receive intravenous remdesivir for either 5 days or 10 days. All patients received 200 mg of remdesivir on day 1 and 100 mg once daily on subsequent days. The primary end point was clinical status on day 14, assessed on a 7-point ordinal scale.
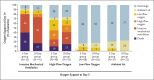
Results: In total, 397 patients underwent randomization and began treatment (200 patients for 5 days and 197 for 10 days). The median duration of treatment was 5 days (interquartile range, 5 to 5) in the 5-day group and 9 days (interquartile range, 5 to 10) in the 10-day group. At baseline, patients randomly assigned to the 10-day group had significantly worse clinical status than those assigned to the 5-day group (P = 0.02). By day 14, a clinical improvement of 2 points or more on the ordinal scale occurred in 64% of patients in the 5-day group and in 54% in the 10-day group. After adjustment for baseline clinical status, patients in the 10-day group had a distribution in clinical status at day 14 that was similar to that among patients in the 5-day group (P = 0.14). The most common adverse events were nausea (9% of patients), worsening respiratory failure (8%), elevated alanine aminotransferase level (7%), and constipation (7%).
Conclusions: In patients with severe Covid-19 not requiring mechanical ventilation, our trial did not show a significant difference between a 5-day course and a 10-day course of remdesivir. With no placebo control, however, the magnitude of benefit cannot be determined. (Funded by Gilead Sciences; GS-US-540-5773 ClinicalTrials.gov number, NCT04292899.).
Copyright © 2020 Massachusetts Medical Society.
Figures
Comment in
-
Remdesivir - An Important First Step.N Engl J Med. 2020 Nov 5;383(19):1886-1887. doi: 10.1056/NEJMe2018715. Epub 2020 May 27. N Engl J Med. 2020. PMID: 32459913 Free PMC article. No abstract available.
References
-
- Abi-Habib M. Millions had risen out of poverty. Coronavirus is pulling them back. New York Times. April 30, 2020. (https://www.nytimes.com/2020/04/30/world/asia/coronavirus-poverty-unempl...).
-
- Romm T. Mass layoffs begin in cities and states amid coronavirus fallout, threatening education, sanitation, health and safety. Washington Post. April 29, 2020. (https://www.washingtonpost.com/business/2020/04/29/cities-states-layoffs...).
-
- Johns Hopkins Coronavirus Resource Center home page (https://coronavirus.jhu.edu/).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
