Biomarker-Guided Development of DNA Repair Inhibitors
- PMID: 32459988
- PMCID: PMC7316088
- DOI: 10.1016/j.molcel.2020.04.035
Biomarker-Guided Development of DNA Repair Inhibitors
Abstract
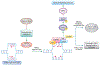
Anti-cancer drugs targeting the DNA damage response (DDR) exploit genetic or functional defects in this pathway through synthetic lethal mechanisms. For example, defects in homologous recombination (HR) repair arise in cancer cells through inherited or acquired mutations in BRCA1, BRCA2, or other genes in the Fanconi anemia/BRCA pathway, and these tumors have been shown to be particularly sensitive to inhibitors of the base excision repair (BER) protein poly (ADP-ribose) polymerase (PARP). Recent work has identified additional genomic and functional assays of DNA repair that provide new predictive and pharmacodynamic biomarkers for these targeted therapies. Here, we examine the development of selective agents targeting DNA repair, including PARP inhibitors; inhibitors of the DNA damage kinases ataxia-telangiectasia and Rad3 related (ATR), CHK1, WEE1, and ataxia-telangiectasia mutated (ATM); and inhibitors of classical non-homologous end joining (cNHEJ) and alternative end joining (Alt EJ). We also review the biomarkers that guide the use of these agents and current clinical trials with these therapies.
Keywords: DNA repair; PARP inhibitor; cell-cycle kinases; polymerase theta.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests J.M.C. received research funding from Merck, AstraZeneca, and Tesaro; has served as a consultant to Bristol Myers Squib; and received travel funding from Bristol Myers Squib and Roche. A.J.A. has consulted for Oncorus, Arrakis Therapeutics, and Merck & Co. and has research funding from Mirati Therapeutics and Deerfield that are unrelated to this project. G.I.S. has received research funding from Eli Lilly, Merck KGaA/EMD-Serono, Merck, and Sierra Oncology; has served on advisory boards for Pfizer, Eli Lilly, G1 Therapeutics, Roche, Merck KGaA/EMD-Serono, Sierra Oncology, Bicycle Therapeutics, Fusion Pharmaceuticals, Cybrexa Therapeutics, Astex, Almac, Ipsen, Bayer, Angiex, Daiichi Sankyo, Seattle Genetics, Boehringer Ingelheim, ImmunoMet, Asana, Artios, Atrin, and Concarlo Holdings; and holds a patent entitled, “Dosage regimen for sapacitabine and seliciclib,” also issued to Cyclacel Pharmaceuticals, and a pending patent entitled, “Compositions and Methods for Predicting Response and Resistance to CDK4/6 Inhibition,” together with Liam Cornell. A.D.D. is a consultant/advisory board member for Lilly Oncology, Merck-EMD Serono, Intellia Therapeutics, Sierra Oncology, Cyteir Therapeutics, Third Rock Ventures, AstraZeneca, Ideaya, and Cedilla Therapeutics; a stockholder in Ideaya, Cedilla Therapeutics, and Cyteir; and reports receiving commercial research grants from Lilly Oncology and Merck-EMD Serono.
Figures

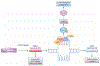
References
-
- Ang JE, Gourley C, Powell CB, High H, Shapira-Frommer R, Castonguay V, De Greve J, Atkinson T, Yap TA, Sandhu S, et al. (2013). Efficacy of chemotherapy in BRCA1/2 mutation carrier ovarian cancer in the setting of PARP inhibitor resistance: a multi-institutional study. Clinical cancer research : an official journal of the American Association for Cancer Research 19, 5485–5493. - PubMed
-
- Banerji U, Plummer ER, Moreno V, Ang JE, Quinton A, Drew Y, Hernandez T, Roda D, Carter L, Navarro A, et al. (2019). A phase I/II first-in-human trial of oral SRA737 (a Chk1 inhibitor) given in combination with low-dose gemcitabine in subjects with advanced cancer. Journal of Clinical Oncology 37, 3095–3095.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

