A Comprehensive Integrated Anatomical and Molecular Atlas of Rat Intrinsic Cardiac Nervous System
- PMID: 32460006
- PMCID: PMC7327996
- DOI: 10.1016/j.isci.2020.101140
A Comprehensive Integrated Anatomical and Molecular Atlas of Rat Intrinsic Cardiac Nervous System
Abstract
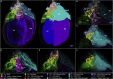
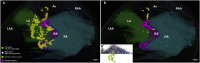
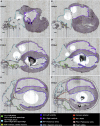
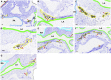
We have developed and integrated several technologies including whole-organ imaging and software development to support an initial precise 3D neuroanatomical mapping and molecular phenotyping of the intracardiac nervous system (ICN). While qualitative and gross anatomical descriptions of the anatomy of the ICN have each been pursued, we here bring forth a comprehensive atlas of the entire rat ICN at single-cell resolution. Our work precisely integrates anatomical and molecular data in the 3D digitally reconstructed whole heart with resolution at the micron scale. We now display the full extent and the position of neuronal clusters on the base and posterior left atrium of the rat heart, and the distribution of molecular phenotypes that are defined along the base-to-apex axis, which had not been previously described. The development of these approaches needed for this work has produced method pipelines that provide the means for mapping other organs.
Keywords: Cellular Neuroscience; Imaging Anatomy; Molecular Neuroscience; Rodent Cardiology; Small Animal Imaging; Transcriptomics.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests S.T. and M.H. are paid employees of MBF Bioscience (Williston, VT). S.T. and M.H. are also funded by the NIH Common Fund award, OT3OD025349 to Dr. Shivkumar at University of California Los Angeles (subaward to J.S.S., R.V., J.C.), to create multi-scale, multi-organ, multi-species SPARC map management as a part of SPARC Portal. The software development efforts described in this manuscript preceded integration with SPARC DRC. Owing to intellectual property right restrictions, we cannot provide the Tissue Mapper, Tissue Maker or Biolucida Converter source code or its documentation at this time. Strateos and MBF Bioscience are commercial entities, and the authors affiliated with them are company employees. The remaining authors declare that no competing interests exist.
Figures










References
-
- Ai J., Gozal D., Li L., Wead W.B., Chapleau M.W., Wurster R., Yang B., Li H., Liu R., Cheng Z. Degeneration of vagal efferent axons and terminals in cardiac ganglia of aged rats. J. Comp. Neurol. 2007;504:74–88. - PubMed
-
- Allard M., Labrouche S., Nosjean A., Laguzzi R. Mechanisms underlying the cardiovascular responses to peripheral administration of NPFF in the rat. J. Pharmacol. Exp. Ther. 1995;274:577–583. - PubMed
-
- Armour J.A., Murphy D.A., Yuan B.X., Macdonald S., Hopkins D.A. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat. Rec. 1997;247:289–298. - PubMed
-
- Arora R.C., Cardinal R., Smith F.M., Ardell J.L., Dell’Italia L.J., Armour J.A. Intrinsic cardiac nervous system in tachycardia induced heart failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R1212–R1223. - PubMed
-
- Beaumont E., Salavatian S., Southerland E.M., Vinet A., Jacquemet V., Armour J.A., Ardell J.L. Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function. J. Physiol. (Lond) 2013;591:4515–4533. doi: 10.1113/jphysiol.2013.259382. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

