SIX2 Regulates Human β Cell Differentiation from Stem Cells and Functional Maturation In Vitro
- PMID: 32460030
- PMCID: PMC7304247
- DOI: 10.1016/j.celrep.2020.107687
SIX2 Regulates Human β Cell Differentiation from Stem Cells and Functional Maturation In Vitro
Abstract
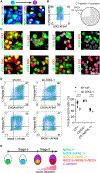
Generation of insulin-secreting β cells in vitro is a promising approach for diabetes cell therapy. Human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs) are differentiated to β cells (SC-β cells) and mature to undergo glucose-stimulated insulin secretion, but molecular regulation of this defining β cell phenotype is unknown. Here, we show that maturation of SC-β cells is regulated by the transcription factor SIX2. Knockdown (KD) or knockout (KO) of SIX2 in SC-β cells drastically limits glucose-stimulated insulin secretion in both static and dynamic assays, along with the upstream processes of cytoplasmic calcium flux and mitochondrial respiration. Furthermore, SIX2 regulates the expression of genes associated with these key β cell processes, and its expression is restricted to endocrine cells. Our results demonstrate that expression of SIX2 influences the generation of human SC-β cells in vitro.
Keywords: SIX2; beta cells; diabetes; differentiation; insulin; islets; pluripotent stem cells; stem cells.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests L.V.-C., N.J.H., and J.R.M. are inventors on patent applications relating to the differentiation procedure.
Figures


References
-
- Cardenas-Diaz FL, Osorio-Quintero C, Diaz-Miranda MA, Kishore S, Leavens K, Jobaliya C, Stanescu D, Ortiz-Gonzalez X, Yoon C, Chen CS, et al. (2019). Modeling Monogenic Diabetes using Human ESCs Reveals Developmental and Metabolic Deficiencies Caused by Mutations in HNF1A. Cell Stem Cell 25, 273–289.e5. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

