Patient-derived pancreatic tumour organoids identify therapeutic responses to oncolytic adenoviruses
- PMID: 32460166
- PMCID: PMC7251378
- DOI: 10.1016/j.ebiom.2020.102786
Patient-derived pancreatic tumour organoids identify therapeutic responses to oncolytic adenoviruses
Abstract
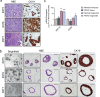
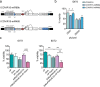
Background: Pancreatic patient-derived organoids (PDOs) are a well-established model for studying pancreatic ductal adenocarcinoma (PDAC) carcinogenesis and are potential predictors of clinical responses to chemotherapy. Oncolytic virotherapy is envisioned as a novel treatment modality for pancreatic cancer, and candidate viruses are being tested in clinical trials. Here, we explore the feasibility of using PDOs as a screening platform for the oncolytic adenovirus (OA) response.
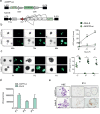
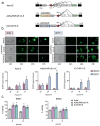
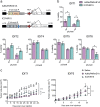
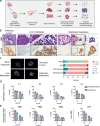
Methods: Organoids were established from healthy pancreas and PDAC tissues and assessed for infectivity, oncoselectivity, and patient-dependent sensitivity to OA. Antitumour effects were studied in vivo in organoid xenografts. Further evaluation of oncolytic responses was conducted in organoids derived from orthotopic models or metastastic tissues.
Findings: Oncolytic adenoviruses display good selectivity, with replication only in organoids derived from PDAC tumours. Furthermore, responses of PDOs to a set of OAs reveal individual differences in cytotoxicity as well as in synergism with standard chemotherapy. Adenoviral cytotoxicity in PDOs is predictive of antitumour efficacy in a subcutaneous xenograft setting. Organoids from orthotopic tumours and metastases in nude mice mirror the viral preference of PDOs, indicating that PDO sensitivity to OAs could be informative about responses in both primary tumours and metastatic foci.
Interpretation: Our data imply that pancreatic PDOs can serve as predictive tools for screening for sensitivity to OA.
Keywords: Oncolytic adenovirus (OA); Orthotopic tumours; Pancreatic ductal adenocarcinoma (PDAC); Patient-derived organoids (PDO).
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest SFB and HC declare that they hold a patent (WO2015/173425). All other authors declare no conflict of interest.
Figures
Comment in
-
Oncolytic virotherapy meets the human organoid technology for pancreatic cancers.EBioMedicine. 2020 Jul;57:102828. doi: 10.1016/j.ebiom.2020.102828. Epub 2020 Jun 20. EBioMedicine. 2020. PMID: 32574953 Free PMC article. No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

