Longevity genes, cardiac ageing, and the pathogenesis of cardiomyopathy: implications for understanding the effects of current and future treatments for heart failure
- PMID: 32460327
- PMCID: PMC7599035
- DOI: 10.1093/eurheartj/ehaa360
Longevity genes, cardiac ageing, and the pathogenesis of cardiomyopathy: implications for understanding the effects of current and future treatments for heart failure
Abstract
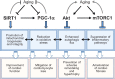
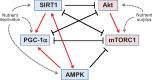
The two primary molecular regulators of lifespan are sirtuin-1 (SIRT1) and mammalian target of rapamycin complex 1 (mTORC1). Each plays a central role in two highly interconnected pathways that modulate the balance between cellular growth and survival. The activation of SIRT1 [along with peroxisome proliferator-activated receptor-gamma coactivator (PGC-1α) and adenosine monophosphate-activated protein kinase (AMPK)] and the suppression of mTORC1 (along with its upstream regulator, Akt) act to prolong organismal longevity and retard cardiac ageing. Both activation of SIRT1/PGC-1α and inhibition of mTORC1 shifts the balance of cellular priorities so as to promote cardiomyocyte survival over growth, leading to cardioprotective effects in experimental models. These benefits may be related to direct actions to modulate oxidative stress, organellar function, proinflammatory pathways, and maladaptive hypertrophy. In addition, a primary shared benefit of both SIRT1/PGC-1α/AMPK activation and Akt/mTORC1 inhibition is the enhancement of autophagy, a lysosome-dependent degradative pathway, which clears the cytosol of dysfunctional organelles and misfolded proteins that drive the ageing process by increasing oxidative and endoplasmic reticulum stress. Autophagy underlies the ability of SIRT1/PGC-1α/AMPK activation and Akt/mTORC1 suppression to extend lifespan, mitigate cardiac ageing, alleviate cellular stress, and ameliorate the development and progression of cardiomyopathy; silencing of autophagy genes abolishes these benefits. Loss of SIRT1/PGC-1α/AMPK function or hyperactivation of Akt/mTORC1 is a consistent feature of experimental cardiomyopathy, and reversal of these abnormalities mitigates the development of heart failure. Interestingly, most treatments that have been shown to be clinically effective in the treatment of chronic heart failure with a reduced ejection fraction have been reported experimentally to exert favourable effects to activate SIRT1/PGC-1α/AMPK and/or suppress Akt/mTORC1, and thereby, to promote autophagic flux. Therefore, the impairment of autophagy resulting from derangements in longevity gene signalling is likely to represent a seminal event in the evolution and progression of cardiomyopathy.
Keywords: Adenosine monophophase-activated protein kinase; Akt/mTOR pathway; Cardiac ageing; Heart failure; Sirtuin-1.
© The Author(s) 2020. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures


Similar articles
-
Autophagy-dependent and -independent modulation of oxidative and organellar stress in the diabetic heart by glucose-lowering drugs.Cardiovasc Diabetol. 2020 May 13;19(1):62. doi: 10.1186/s12933-020-01041-4. Cardiovasc Diabetol. 2020. PMID: 32404204 Free PMC article. Review.
-
Qiliqiangxin: A multifaceted holistic treatment for heart failure or a pharmacological probe for the identification of cardioprotective mechanisms?Eur J Heart Fail. 2023 Dec;25(12):2130-2143. doi: 10.1002/ejhf.3068. Epub 2023 Nov 14. Eur J Heart Fail. 2023. PMID: 37877337 Review.
-
Regulation of diabetic cardiomyopathy by caloric restriction is mediated by intracellular signaling pathways involving 'SIRT1 and PGC-1α'.Cardiovasc Diabetol. 2018 Aug 2;17(1):111. doi: 10.1186/s12933-018-0754-4. Cardiovasc Diabetol. 2018. PMID: 30071860 Free PMC article.
-
The Role of Heme Oxygenase 1 in the Protective Effect of Caloric Restriction against Diabetic Cardiomyopathy.Int J Mol Sci. 2019 May 16;20(10):2427. doi: 10.3390/ijms20102427. Int J Mol Sci. 2019. PMID: 31100876 Free PMC article.
-
CTRP6-mediated cardiac protection in heart failure via the AMPK/SIRT1/PGC-1α signalling pathway.Exp Physiol. 2024 Dec;109(12):2031-2045. doi: 10.1113/EP092036. Epub 2024 Sep 26. Exp Physiol. 2024. PMID: 39325807 Free PMC article.
Cited by
-
GLP-1 Analogs, SGLT-2, and DPP-4 Inhibitors: A Triad of Hope for Alzheimer's Disease Therapy.Biomedicines. 2023 Nov 12;11(11):3035. doi: 10.3390/biomedicines11113035. Biomedicines. 2023. PMID: 38002034 Free PMC article. Review.
-
Diosmetin Protects against Cardiac Hypertrophy via p62/Keap1/Nrf2 Signaling Pathway.Oxid Med Cell Longev. 2022 Feb 22;2022:8367997. doi: 10.1155/2022/8367997. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35242278 Free PMC article.
-
Echocardiographic phenotypes of Chinese patients with type 2 diabetes may indicate early diabetic myocardial disease.ESC Heart Fail. 2022 Oct;9(5):3327-3344. doi: 10.1002/ehf2.14062. Epub 2022 Jul 13. ESC Heart Fail. 2022. PMID: 35831174 Free PMC article.
-
Inhibition of miR-199a-5p rejuvenates aged mesenchymal stem cells derived from patients with idiopathic pulmonary fibrosis and improves their therapeutic efficacy in experimental pulmonary fibrosis.Stem Cell Res Ther. 2021 Feb 25;12(1):147. doi: 10.1186/s13287-021-02215-x. Stem Cell Res Ther. 2021. PMID: 33632305 Free PMC article.
-
Qishen Yiqi dropping pills improve isoproterenol-induced cardiomyocyte hypertrophy by regulating X-inactive specific transcript (XIST) expression in rats.J Thorac Dis. 2022 Jun;14(6):2213-2223. doi: 10.21037/jtd-22-606. J Thorac Dis. 2022. PMID: 35813728 Free PMC article.
References
-
- Ma L, Dong W, Wang R, Li Y, Xu B, Zhang J, Zhao Z, Wang Y. Effect of caloric restriction on the SIRT1/mTOR signaling pathways in senile mice. Brain Res Bull 2015;116:67–72. - PubMed
-
- Meijles DN, Zoumpoulidou G, Markou T, Rostron KA, Patel R, Lay K, Handa BS, Wong B, Sugden PH, Clerk A. The cardiomyocyte “redox rheostat”: redox signalling via the AMPK-mTOR axis and regulation of gene and protein expression balancing survival and death. J Mol Cell Cardiol 2019;129:118–129. - PMC - PubMed
-
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 2000;289:2126–2128. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

