A Randomized, Double-Blind, Placebo-Controlled Trial of Vilazodone in Children and Adolescents with Major Depressive Disorder with Twenty-Six-Week Open-Label Follow-Up
- PMID: 32460523
- PMCID: PMC7409584
- DOI: 10.1089/cap.2019.0176
A Randomized, Double-Blind, Placebo-Controlled Trial of Vilazodone in Children and Adolescents with Major Depressive Disorder with Twenty-Six-Week Open-Label Follow-Up
Abstract
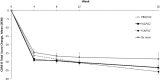
Objective: To evaluate the efficacy and long-term safety of vilazodone in children and adolescent outpatients with major depressive disorder (MDD). Methods: Children and adolescents aged 7-17 years of age with MDD were randomized 2:2:1 to 8 weeks of double-blind placebo, vilazodone 15 or 30 mg/day or fluoxetine 20 mg/day, respectively. The primary and secondary efficacy outcomes, respectively, were change from baseline to week 8 in Children's Depression Rating Scale-Revised (CDRS-R) score total score and Clinical Global Impressions-Severity (CGI-S) score analyzed using a mixed model for repeated measurement approach. Patients who completed the 8-week randomized controlled trial (RCT), as well as new (de novo) patients, could participate in a 26-week, vilazodone-only, open-label extension (OLE) study. Results: The RCT enrolled 473 patients (60% female) with an average age of 13 years. Change in CDRS-R and CGI-S scores from baseline to week 8 did not differ between patients who received vilazodone and those randomized to placebo. The least-squares mean change from baseline in CDRS-R scores was similar for vilazodone and placebo (-20.7 vs. -20.3, p = 0.77; least-squares mean difference [LSMD] = -0.40). For fluoxetine, the LSMD versus placebo was -2.3 (p = 0.14). The OLE enrolled 330 patients (60% female) with an average age of 13-14 years. Overall, no new safety concerns were identified compared to what is known in adults. Conclusions: Similar improvements in depressive symptoms were observed in all arms. This study does not support the efficacy of vilazodone 15 or 30 mg/day for pediatric patients with MDD. No new or unexpected safety concerns were detected during the RCT or OLE studies.
Keywords: adolescents; children; clinical trial; major depressive disorder; treatment efficacy; vilazodone.
Figures

References
-
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000
-
- Atkinson SD, Prakash A, Zhang Q, Pangallo BA, Bangs ME, Emslie GJ, March JS: A double-blind efficacy and safety study of duloxetine flexible dosing in children and adolescents with major depressive disorder. J Child Adolesc Psychopharmacol 24:180–189, 2014 - PubMed
-
- Bridge JA, Birmaher B, Iyengar S, Barbe RP, Brent DA: A placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. Am J Psychiatry 166:42–49, 2009 - PubMed
-
- Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, Ren L, Brent DA: Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: A meta-analysis of randomized controlled trials. JAMA 297:1683–1696, 2007 - PubMed
-
- Cohen D, Consoli A, Bodeau N, Purper-Ouakil D, Deniau E, Guile JM, Donnelly C: Predictors of placebo response in randomized controlled trials of psychotropic drugs for children and adolescents with internalizing disorders. J Child Adolesc Psychopharmacol 20:39–47, 2010 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
