The Repeatability of Inspiration Performance Through Different Inhalers in Patients with Chronic Obstructive Pulmonary Disease and Control Volunteers
- PMID: 32460588
- PMCID: PMC7526298
- DOI: 10.1089/jamp.2020.1594
The Repeatability of Inspiration Performance Through Different Inhalers in Patients with Chronic Obstructive Pulmonary Disease and Control Volunteers
Abstract
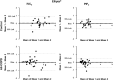
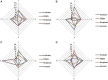
Background: Inhalation therapy is a cornerstone of treating patients with chronic obstructive pulmonary disease (COPD). Inhaler types and through-device inhalation parameters influence airway drug delivery. We aimed to measure the repeatability of inhalation performance through four different commercially available inhalers. Methods: We recruited control subjects (n = 22) and patients with stable COPD (S-COPD, n = 16) and during an acute exacerbation (AE-COPD, n = 15). Standard spirometry was followed by through-device inhalation maneuvers using Ellipta®, Evohaler®, Respimat®, and Genuair®. Through-device inspiratory vital capacity (IVCd) and peak inspiratory flow (PIFd), as well as inhalation time (tin) and breath hold time (tbh), were recorded and all measurements were repeated in a random manner. Results: There was no difference in forced expiratory volume in 1 second (FEV1) between patients (S-COPD: 39 ± 5 vs. AE-COPD: 32% ± 5% predicted, p > 0.05). In controls, the IVCd was significantly reduced by all four devices in comparison with the slight reduction seen in COPD patients. In all subjects, PIF was lowered when inhaling through the devices in order of decreasing magnitude in PIFd: Evohaler, Respimat, Ellipta, and Genuair. The Bland-Altman analysis showed a highly variable coefficient of repeatability for IVCd and PIFd through the different inhalers for all COPD patients. Based on the intermeasurement differences in patients, Respimat and Genuair showed the highest repeatability for IVCd, while Genuair and Ellipta performed superior with regard to PIFd. Conclusions: Our study is the first to compare repeatability of inhalation performances through different inhalers in COPD patients, showing great individual differences for parameters influencing lung deposition of inhaled medication from a given device. Our data provide new insight into the characterization of inhaler use by patients with COPD, and might aid the selection of the most appropriate devices to ensure the adequate and consistent delivery of inhaled drugs.
Keywords: COPD; exacerbation; inhalation device; inhaled therapy; repeatability.
Conflict of interest statement
M.V. and T.L. received consultation fees from Chiesi Hungary, Berlin Chemie Menarini, Boehringer Ingelheim, and Glaxo Smith Kline. All other authors have no conflict of interest to declare.
Figures



References
-
- GOLD. Global Strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: Available from: wwwgoldcopdorg, 2019
-
- Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, Dransfield MT, Halpin DMG, Han MK, Jones CE, Kilbride S, Lange P, Lomas DA, Martinez FJ, Singh D, Tabberer M, Wise RA, and Pascoe SJ: Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378:1671–1680 - PubMed
-
- Papi A, Vestbo J, Fabbri L, Corradi M, Prunier H, Cohuet G, Guasconi A, Montagna I, Vezzoli S, Petruzzelli S, Scuri M, Roche N, and Singh D: Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): A double-blind, parallel group, randomised controlled trial. Lancet. 2018;391:1076–1084 - PubMed
-
- Haidl P, Heindl S, Siemon K, Bernacka M, and Cloes RM: Inhalation device requirements for patients' inhalation maneuvers. Respir Med. 2016;118:65–75 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

