Infection with brainworm (Elaphostrongylus rangiferi) in reindeer (Rangifer tarandus ssp.) in Fennoscandia
- PMID: 32460832
- PMCID: PMC7254673
- DOI: 10.1186/s13028-020-00524-4
Infection with brainworm (Elaphostrongylus rangiferi) in reindeer (Rangifer tarandus ssp.) in Fennoscandia
Abstract
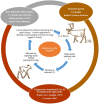
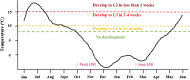
Sami reindeer herders have considerable traditional knowledge about a neurological reindeer disease resembling elaphostrongylosis, but the causative agent was not identified prior to the description of the brainworm Elaphostrongylus rangiferi in Russia in 1958. Elaphostrongylosis was quickly recognised as a serious cause of reindeer morbidity and mortality. The ecology, epidemiology and pathophysiology of the disease were studied in Sweden and Norway during the 1960s and in particular the 1970s to 1990s. In Finland, elaphostrongylosis was not recognised as an important disease for Finnish reindeer husbandry, even though the presence of brainworm infection has been documented. Brainworm has an indirect lifecycle with snail and slug intermediate hosts. The free-living L1 larvae have extremely good freeze tolerance and can survive > 360 days at - 80 °C in water (solid ice). Even though reindeer brainworm is clearly well adapted to the Arctic chill, the lifecycle stages outside the reindeer final host are sped up at warmer environmental temperatures. Arctic summer temperatures are close to the developmental threshold of the parasite in the intermediate gastropod hosts (8-10 °C), and the parasite has typically had a 2-year life cycle. Disease outbreaks generally occur during the winter following the infection of reindeer with infected snails and slugs during the summer and autumn. Warmer summers result in faster development of brainworm larvae in the intermediate hosts. Clinical symptoms have been seen reported as early as August, such as in the outbreak in Trøndelag, Norway in 2018. The reindeer brainworm is also a cause of conflict between reindeer herders and small ruminant farmers, because it can cause severe disease in goats and sheep, which share pasture with reindeer. Many knowledge gaps remain if we wish to successfully predict and mitigate for large-scale outbreaks in a future with a predicted warmer, wetter and wilder climate.
Keywords: Climate change; Diagnosis; Epizootic; Goat; Lifecycle; Neurological disease; Pathogenesis; Protostrongylid; Rangifer tarandus tarandus; Sheep; Treatment.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
-
- Josefsen T, Handeland K. Brainworm (Elaphostrongylus rangiferi) in reindeer—lifecycle and veterinary considerations. Nor Vet Tidsskr. 2014;126:202–208.
-
- Lankester MW. Chapter 9: extrapulmonary lungworms in cervids. In: Samuel WM, Pybus MJ, Kocan AA, editors. Parasitic diseases of wildlife mammals. Iowa: Iowa State University Press Ames; 2001. pp. 279–300.
-
- Mitskevich VY. Lifecycle of Elaphostrongylus rangiferi Miz. 1958 (in Russian). In: Boev SN, editor. Parasites of farm animals in Kazakhstan. Izdatel: Akademii Nauk SSSR Alma-Ata; 1964, p. 49–60.
-
- Røed K, Côté S, Yannic G. Rangifer tarandus: classification and genetic variation. In: Tryland M, Kutz SJ, editors. Reindeer and Caribou Health and Disease. Boca Raton: CRC Press; 2019. pp. 3–13.
-
- Riseth JÅ, Tømmervik H, Forbes BC. Sustainable and resilient reindeer herding. In: Tryland M, Kutz SJ, editors. Reindeer and Caribou Health and Disease. Boca Raton: CRC Press; 2019. pp. 23–43.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

