Intravenous infusion of human umbilical cord Wharton's jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia
- PMID: 32460839
- PMCID: PMC7251558
- DOI: 10.1186/s13287-020-01725-4
Intravenous infusion of human umbilical cord Wharton's jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia
Abstract
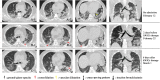
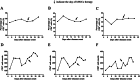
The novel coronavirus disease 2019 (COVID-19) has grown to be a global public health emergency since patients were first detected in Wuhan, China. Thus far, no specific drugs or vaccines are available to cure the patients with COVID-19 infection. The immune system and inflammation are proposed to play a central role in COVID-19 pathogenesis. Mesenchymal stem cells (MSCs) have been shown to possess a comprehensive powerful immunomodulatory function. Intravenous infusion of MSCs has shown promising results in COVID-19 treatment. Here, we report a case of a severe COVID-19 patient treated with human umbilical cord Wharton's jelly-derived MSCs (hWJCs) from a healthy donor in Liaocheng People's Hospital, China, from February 24, 2020. The pulmonary function and symptoms of the patient with COVID-19 pneumonia was significantly improved in 2 days after hWJC transplantation, and recovered and discharged in 7 days after treatment. After treatment, the percentage and counts of lymphocyte subsets (CD3+, CD4+, and CD8+ T cell) were increased, and the level of IL-6, TNF-α, and C-reactive protein is significantly decreased after hWJC treatment. Thus, the intravenous transplantation of hWJCs was safe and effective for the treatment of patients with COVID-19 pneumonia, especially for the patients in a critically severe condition. This report highlights the potential of hWJC infusions as an effective treatment for COVID-19 pneumonia.
Keywords: COVID-19; Human umbilical cord Wharton’s jelly-derived MSCs; Immunomodulatory; Treatment.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
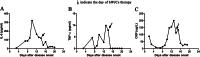
Similar articles
-
Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells.Stem Cell Res Ther. 2020 Aug 18;11(1):361. doi: 10.1186/s13287-020-01875-5. Stem Cell Res Ther. 2020. PMID: 32811531 Free PMC article. Clinical Trial.
-
Expanded Umbilical Cord Mesenchymal Stem Cells (UC-MSCs) as a Therapeutic Strategy in Managing Critically Ill COVID-19 Patients: The Case for Compassionate Use.Pain Physician. 2020 Mar;23(2):E71-E83. Pain Physician. 2020. PMID: 32214286
-
Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial.Signal Transduct Target Ther. 2020 Aug 27;5(1):172. doi: 10.1038/s41392-020-00286-5. Signal Transduct Target Ther. 2020. PMID: 32855385 Free PMC article. Clinical Trial.
-
Mesenchymal Stem Cell Therapy for COVID-19: Present or Future.Stem Cell Rev Rep. 2020 Jun;16(3):427-433. doi: 10.1007/s12015-020-09973-w. Stem Cell Rev Rep. 2020. PMID: 32281052 Free PMC article. Review.
-
Umbilical cord: an allogenic tissue for potential treatment of COVID-19.Hum Cell. 2021 Jan;34(1):1-13. doi: 10.1007/s13577-020-00444-5. Epub 2020 Oct 9. Hum Cell. 2021. PMID: 33033884 Free PMC article. Review.
Cited by
-
Immune Dysfunction and Multiple Treatment Modalities for the SARS-CoV-2 Pandemic: Races of Uncontrolled Running Sweat?Biology (Basel). 2020 Aug 24;9(9):243. doi: 10.3390/biology9090243. Biology (Basel). 2020. PMID: 32846906 Free PMC article. Review.
-
Regenerative therapy by using mesenchymal stem cells-derived exosomes in COVID-19 treatment. The potential role and underlying mechanisms.Regen Ther. 2022 Jun;20:61-71. doi: 10.1016/j.reth.2022.03.006. Epub 2022 Mar 22. Regen Ther. 2022. PMID: 35340407 Free PMC article. Review.
-
Stem cell-based therapy for human diseases.Signal Transduct Target Ther. 2022 Aug 6;7(1):272. doi: 10.1038/s41392-022-01134-4. Signal Transduct Target Ther. 2022. PMID: 35933430 Free PMC article. Review.
-
Virology of SARS-CoV-2 and management of nCOVID-19 utilizing immunomodulation properties of human mesenchymal stem cells-a literature review.Stem Cell Investig. 2021 Nov 10;8:23. doi: 10.21037/sci-2020-040. eCollection 2021. Stem Cell Investig. 2021. PMID: 34917676 Free PMC article. Review.
-
Gender, age and comorbidities as the main prognostic factors in patients with COVID-19 pneumonia.Am J Transl Res. 2020 Oct 15;12(10):6537-6548. eCollection 2020. Am J Transl Res. 2020. PMID: 33194050 Free PMC article.
References
-
- Liang B, Chen JH, Li T, Wu HY, Yang WJ, Li YJ, et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells. 2020. chinaXiv:202002.00084. http://chinaxiv.org/abs/202002.00084. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

